Let’s discuss the question: the lewis structure of n2h2 shows ________.. We summarize all relevant answers in section Q&A of website Myyachtguardian.com in category: Blog MMO. See more related questions in the comments below.
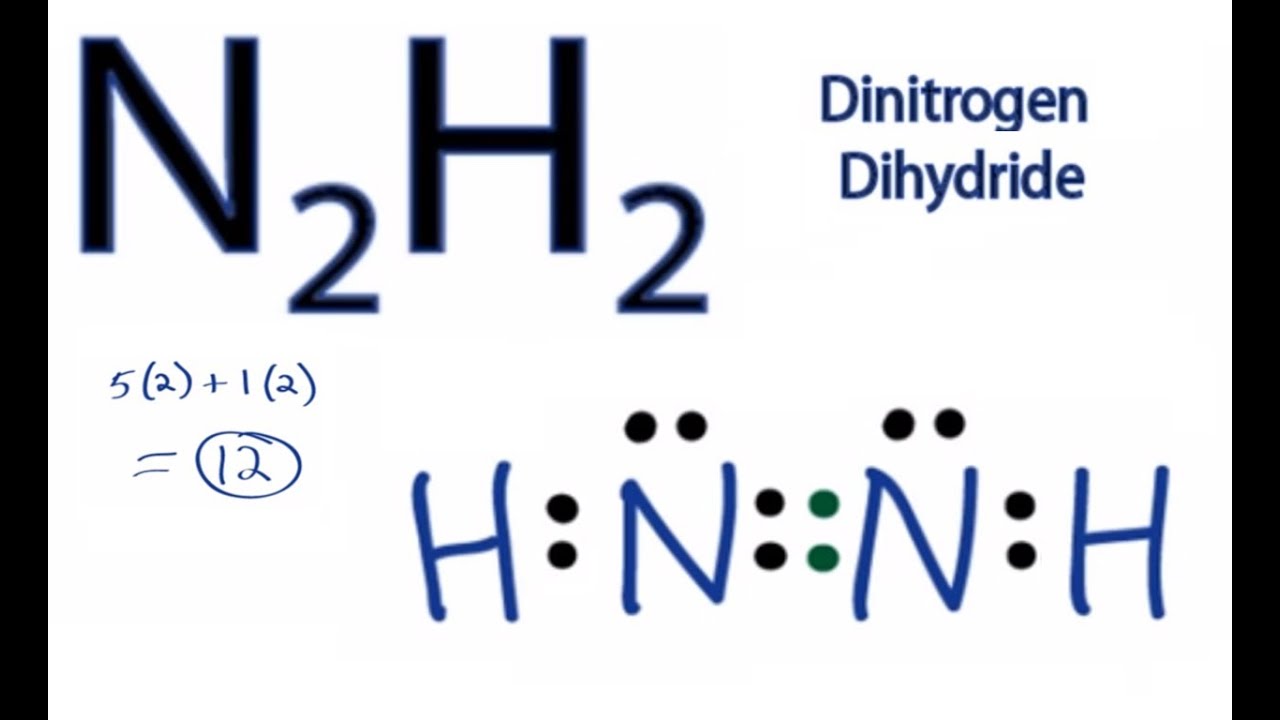
Table of Contents
How many valence electrons does n2h2 have?
In the Lewis structure for N2H2 there are a total of 12 valence electrons. In order to complete the octets on the Nitrogen (N) atoms you will need to form a double bond between the N atoms.
What is the hybridization of N2H2?
In the molecule of N2H2, there are two sigma bonds, one pi bond and a lone pair of electrons around the nitrogen atom. Here again, the lone pair can be considered equivalent to a sigma bond. Hence, the nitrogen atom is sp2 hybridized.
N2H2 Lewis Structure: How to Draw the Lewis Structure for Dinitrogen dihydride
Images related to the topicN2H2 Lewis Structure: How to Draw the Lewis Structure for Dinitrogen dihydride
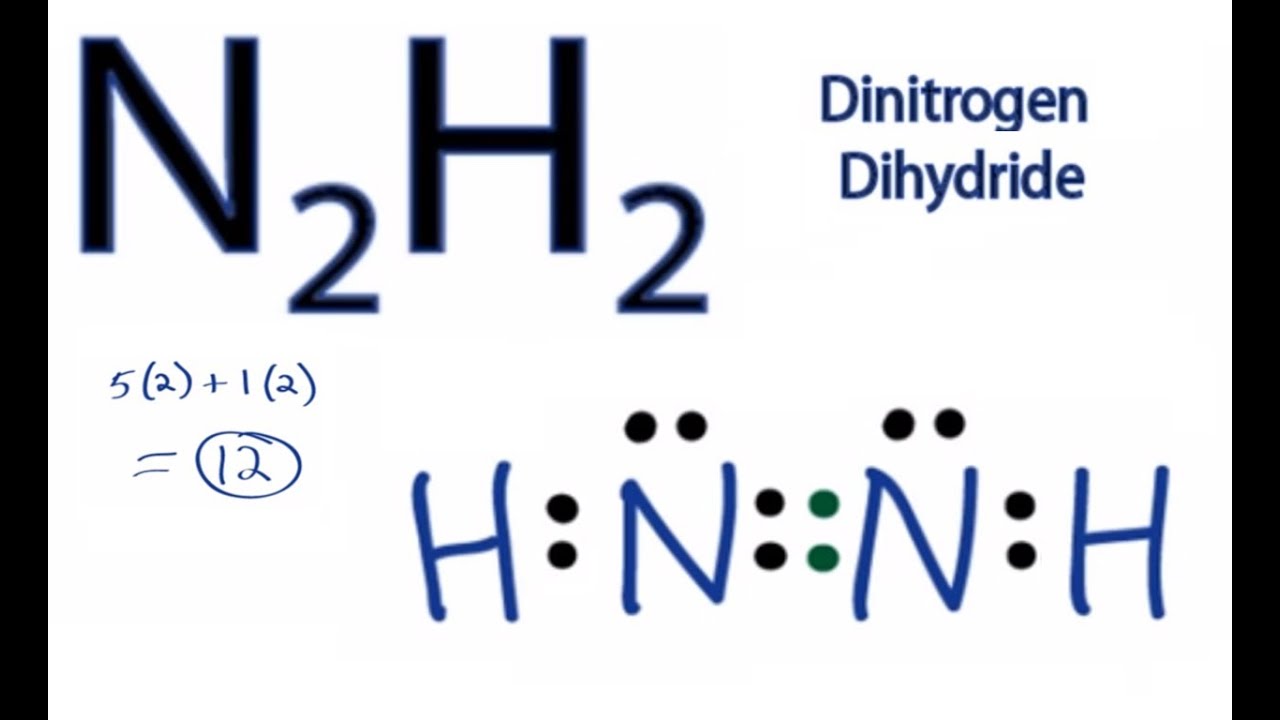
What intermolecular forces are in N2H2?
N2H2 is polar molecule with london dispersion forces,dipole-dipole forces and H-bonding .
Is N2H2 ionic or covalent?
The covalent compound shown is diazene. It contains 2 central nitrogen atoms that are bonded to each other and to 2 hydrogen atoms.
Which Lewis structure is possible for N2O?
The most stable Lewis structure of N2O is represented by option (D). In this structure, more electronegative O atom bears negative charge and less electronegative N atom bears a positive charge. Hence, the charge separation that is as that predicted by electronegativity.
N2H2 Lewis Structure (Dinitrogen Dihydride)
Images related to the topicN2H2 Lewis Structure (Dinitrogen Dihydride)

What type of IMFA is NH3?
In case of NH3, both dipole-dipole intraction and hydrogen bonding are persent as well. you know that hclo intermolecular forces has also dipole-dipole intraction. if you talk about london dispersion forces, it is temporary dipoles, reson is, distribution of electron is not well on molecules.
What is the Lewis structure of methane?
| Lewis structure: | |
| Central atom: | carbon |
| Valence electrons on central atom: | 4 |
| 4 H each contribute 1 electron: | 4 |
| Total: | 8 |
|---|
Is N2H2 a polar molecule?
Answer and Explanation: The molecule is polar. The molecule N2H2 N 2 H 2 is made up of two elements: nitrogen (N) and hydrogen (H).
Is diazene linear or planar?
Would you expect diazine to be a linear molecule (all four atoms on the same line)? Would you expect the molecule to be planar (all four atoms in the same plane)? A Th l l i b th li d l A. The molecule is both linear and planar.
[VIETSUB + ENGSUB] Cách vẽ cấu trúc Lewis phân tử NO2 | Lewis Structure of Nitrogen Dioxide NO2
Images related to the topic[VIETSUB + ENGSUB] Cách vẽ cấu trúc Lewis phân tử NO2 | Lewis Structure of Nitrogen Dioxide NO2
![[Vietsub + Engsub] Cách Vẽ Cấu Trúc Lewis Phân Tử No2 | Lewis Structure Of Nitrogen Dioxide No2](https://i.ytimg.com/vi/BRcmnl0Dozk/maxresdefault.jpg)
What type of compound is N2H2?
Diazene is a nitrogen hydride. It is a conjugate acid of a diazenide.
Is Diazene a compound or mixture?
Diimide, also called diazene or diimine, is a compound having the formula (NH)2. It exists as two geometric isomers, E (trans) and Z (cis).
Related searches
- what shape is n2h2
- of the following species will have bond angles of 120
- the lewis structure of n2h2 shows chegg
- bond enthalpy is
- the lewis structure of pf3 shows that the central phosphorus atom has
- the lewis structure of n2h2 shows
- the molecular geometry of the pf 3 molecule is and this molecule is
- the lewis structure of n2h2 shows quizlet
- In the resonance form of ozone shown below, the formal charge on the central oxygen atom is
- The Lewis structure of PF3 shows that the central phosphorus atom has
- Bond enthalpy is
- what does the lewis structure of n2h2 show
- a valid lewis structure of cannot be drawn without violating the octet rule
- The lewis structure of N2H2 shows
- the molecular geometry of the pf3 molecule is
- in the resonance form of ozone shown below the formal charge on the central oxygen atom is
- A valid Lewis structure of cannot be drawn without violating the octet rule
- what does a lewis structure show
- The molecular geometry of the pf 3 molecule is and this molecule is
- the correct lewis structure of n2h2 shows
- what is the lewis structure for n2h2
Information related to the topic the lewis structure of n2h2 shows ________.
Here are the search results of the thread the lewis structure of n2h2 shows ________. from Bing. You can read more if you want.
You have just come across an article on the topic the lewis structure of n2h2 shows ________.. If you found this article useful, please share it. Thank you very much.

