Let’s discuss the question: how many grams is 5.16 mol br2. We summarize all relevant answers in section Q&A of website Myyachtguardian.com in category: Blog MMO. See more related questions in the comments below.

Table of Contents
How many grams are in a mole of Br2?
…
2.8: The Mole.
| Molecule | Molecular Weight | Mass of 1 Mol of Molecules |
|---|---|---|
| Hg2Br2 | 2(200.59) + 2(79.90) = 560.98 | 560.98 g |
What is the molar mass of 1 mole of Br2?
How To Convert Grams To Moles – VERY EASY!
Images related to the topicHow To Convert Grams To Moles – VERY EASY!

How many grams are in a 1 mole?
…
= 3 x 28.01 = 84.03 grams.
| FORMULAS Related Links | |
|---|---|
| Integral Calculus Formulas List | Orthocentre Of A Triangle Formula |
How do I calculate moles?
- Measure the weight of your substance.
- Use a periodic table to find its atomic or molecular mass.
- Divide the weight by the atomic or molecular mass.
- Check your results with Omni Calculator.
How do you convert from moles to grams?
Multiply the given number of moles (2.50 mol) by the molar mass (122.548 g/mol) to get the grams.
What is the mass of 1 mole of ch4?
How do I calculate molar mass?
Multiply the atomic weight (from the periodic table) of each element by the number of atoms of that element present in the compound. 3. Add it all together and put units of grams/mole after the number. For many (but not all) problems, you can simply round the atomic weights and the molar mass to the nearest 0.1 g/mole.
What is the mass of 2 mol of bromine?
The molar mass of Br2 is 159.808 g/mol. The molar mass of bromine is 79.904 g/mol.
How much is in a mole?
The mole, abbreviated mol, is an SI unit which measures the number of particles in a specific substance. One mole is equal to 6.02214179×1023 atoms, or other elementary units such as molecules.
Are grams and moles the same?
A mole of a substance is equal to as many molecules of that substance as there are atoms of carbon-12 in exactly 12 g of carbon-12. This means that 1 mole of any substance is a weight, in grams, equal to that substance’s molecular weight expressed in atomic mass units.
Converting Between Grams and Moles
Images related to the topicConverting Between Grams and Moles

Why is a mole 6.02 x10 23?
Originally, a mole was the quantity of anything that has the same number of particles found in 12.000 grams of carbon-12. That number of particles is Avogadro’s Number, which is roughly 6.02×1023. A mole of carbon atoms is 6.02×1023 carbon atoms.
How many grams is Cl2?
First we need to determine the mass of one mole of Cl2 (molar mass). Cl2 is made up of 2 chlorine atoms. Each chlorine atom has a molar mass of 35.5 g/mol (from the periodic table). Thus, the molecule Cl2 has a molar mass of 71.0 g/mol.
How many grams are in SN?
The symbol for tin is Sn, and the symbol for grams is g. Therefore, 1 moles of tin to grams is the same as 1 moles of Sn to grams, 1 moles of tin to g, and 1 moles of Sn to g. Furthermore, the atomic mass of tin is 118.69. That means that one mole of tin weighs 118.69 grams (118.69 g/mol).
Is Br2 equal to Avogadro’s number?
Bromine is a diatomic element, therefore, in 1mol of Br2 there is Avogadro’s number molecules of Br2 and there is twice Avogadro’s number atoms of Br .
What is a 1 mole?
A mole is defined as 6.02214076 × 1023 of some chemical unit, be it atoms, molecules, ions, or others. The mole is a convenient unit to use because of the great number of atoms, molecules, or others in any substance.
How many moles are in FE?
Iron has a molar mass of 55.84 grams/mole. So, five grams divided by 55.84 grams/mole gives us 0.089 moles of iron.
How do you find moles from molarity?
To calculate the number of moles in a solution given the molarity, we multiply the molarity by total volume of the solution in liters. How many moles of potassium chloride (KCl) are in 4.0 L of a 0.65 M solution? There are 2.6 moles of KCl in a 0.65 M solution that occupies 4.0 L.
How many moles are in 8 grams of CH4?
We can use the molar mass of methane to calculate the number of moles of methane. The molar mass of hydrogen is 1.0080 grams per mole and the molar mass of carbon is 12.011 grams per mole. The number of moles of methane in 8.0 grams is 0.50 moles.
Stoichiometry Basic Introduction, Mole to Mole, Grams to Grams, Mole Ratio Practice Problems
Images related to the topicStoichiometry Basic Introduction, Mole to Mole, Grams to Grams, Mole Ratio Practice Problems
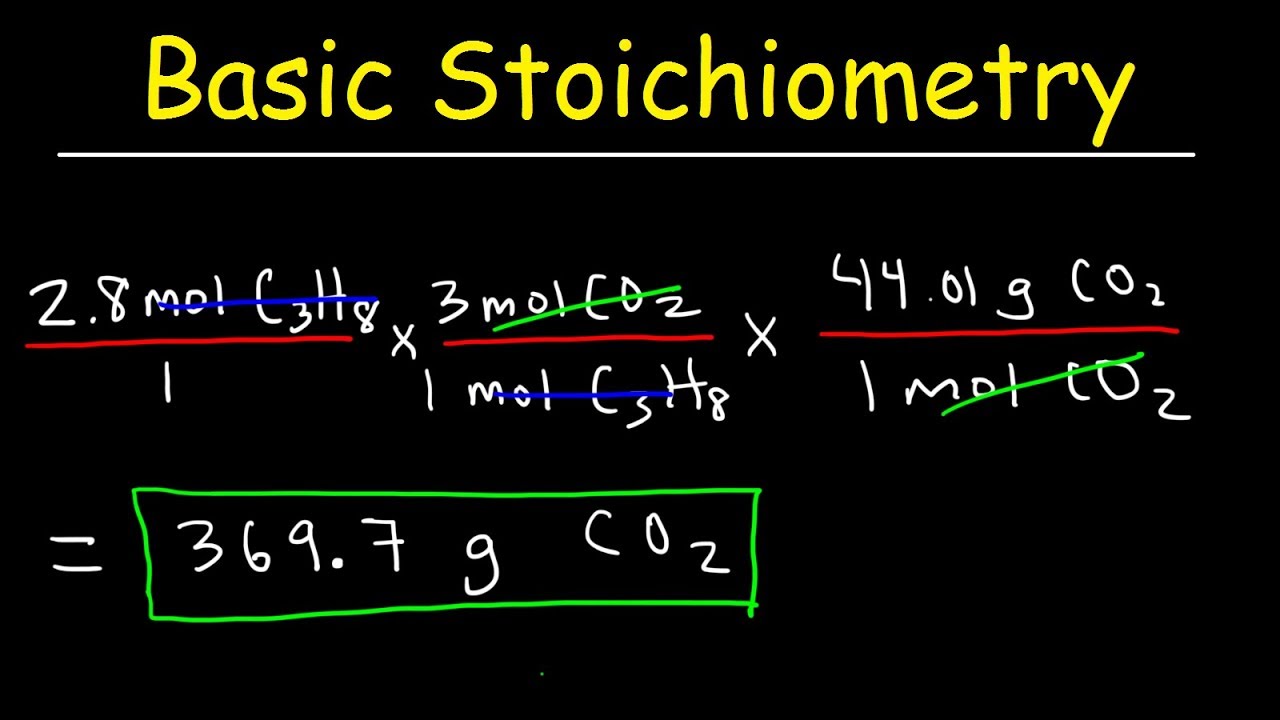
What is the mass of 2 moles of CH4?
1 Answer. Thus, the mass of methane is 32g.
How do you find moles of CH4?
Answer and Explanation: We can convert the mass of methane to the number of moles of methane by using the molar mass of methane as the conversion factor. The calculation can be executed by dividing the mass of methane by its molar mass.
Related searches
- how many grams is 5 16 mol br2 to mol
- how many moles of hcl
- what is the mass of 2 moles of h2
- how many grams is 5 16 mol br2 molar mass
- moles of hydrogen to grams
- how many moles are in 950g of pbso4
- mol of br
- how many grams is 5 16 mol br2 to mg
- how many moles are in 50 grams of kmno4
- grams to moles calculator
- how to convert grams to moles
- how many moles are in 75g of h3po3
Information related to the topic how many grams is 5.16 mol br2
Here are the search results of the thread how many grams is 5.16 mol br2 from Bing. You can read more if you want.
You have just come across an article on the topic how many grams is 5.16 mol br2. If you found this article useful, please share it. Thank you very much.

