Let’s discuss the question: how many molecules are in 0.83 moles of hcl. We summarize all relevant answers in section Q&A of website Myyachtguardian.com in category: Blog MMO. See more related questions in the comments below.

Table of Contents
How many molecules of HCl are in a mole?
One mole of anything is 6.022×1023 of anything, including molecules. Every HCl molecule contains two atoms, H and Cl.
How many molecules is a mole?
The mole is represented by Avogadro’s number, which is 6.022 × 1023 atoms or molecules per mol.
Avogadro’s Number, The Mole, Grams, Atoms, Molar Mass Calculations – Introduction
Images related to the topicAvogadro’s Number, The Mole, Grams, Atoms, Molar Mass Calculations – Introduction

How many molecules are there in HCl?
Note: The number of molecules in a sample is related to moles of compound (1 mol HCl =6.023×1023 HCl molecules). Therefore if you first convert grams HCl to moles, then you can convert moles to number of molecules).
How many molecules are there in 1.5 moles?
Solution. Hence, number of molecules in 1.5 moles of ammonia is 9.033 × 1023.
How many molecules of HCl are present in 2.0 moles of HCl?
So two moles of Hcl will will contain 12.046, multiplied 10 to the power 23 molecules.
How many molecules are in 1g of HCl?
›› More information from the unit converter
The answer is 36.46094. We assume you are converting between grams HCl and mole. You can view more details on each measurement unit: molecular weight of HCl or mol This compound is also known as Hydrochloric Acid.
How many molecules are in 2 moles?
If we have 2 mol of Na atoms, we have 2 × (6.022 × 10 23) Na atoms, or 1.2044 × 10 24 Na atoms. Similarly, if we have 0.5 mol of benzene (C 6H 6) molecules, we have 0.5 × (6.022 × 10 23) C 6H 6 molecules, or 3.011 × 10 23 C 6H 6 molecules.
What is a mole in chemistry?
A mole is a very important unit of measurement that chemists use. A mole of something means you have 602,214,076,000,000,000,000,000 of that thing, like how having a dozen eggs means you have twelve eggs. Chemists have to measure using moles for very small things like atoms, molecules, or other particles.
How do you find moles in chemistry?
If you want to know how many moles of a material you have, divide the mass of the material by its molar mass. The molar mass of a substance is the mass in grams of one mole of that substance. This mass is given by the atomic weight of the chemical unit that makes up that substance in atomic mass units (amu).
How many molecules are in 25g of HCl?
Answer. heya mate! The answer is 36.46094.
How do you convert moles to molecules?
- To go from moles to molecules, multiply the number of moles by 6.02 x 1023.
- To go from molecules to moles, divide the numbers of molecules by 6.02 x 1023.
How many molecules are there in 3.46 g of HCl?
625×1023=0. 628×1023 molecules of Hcl.
Converting Between Moles, Atoms, and Molecules
Images related to the topicConverting Between Moles, Atoms, and Molecules
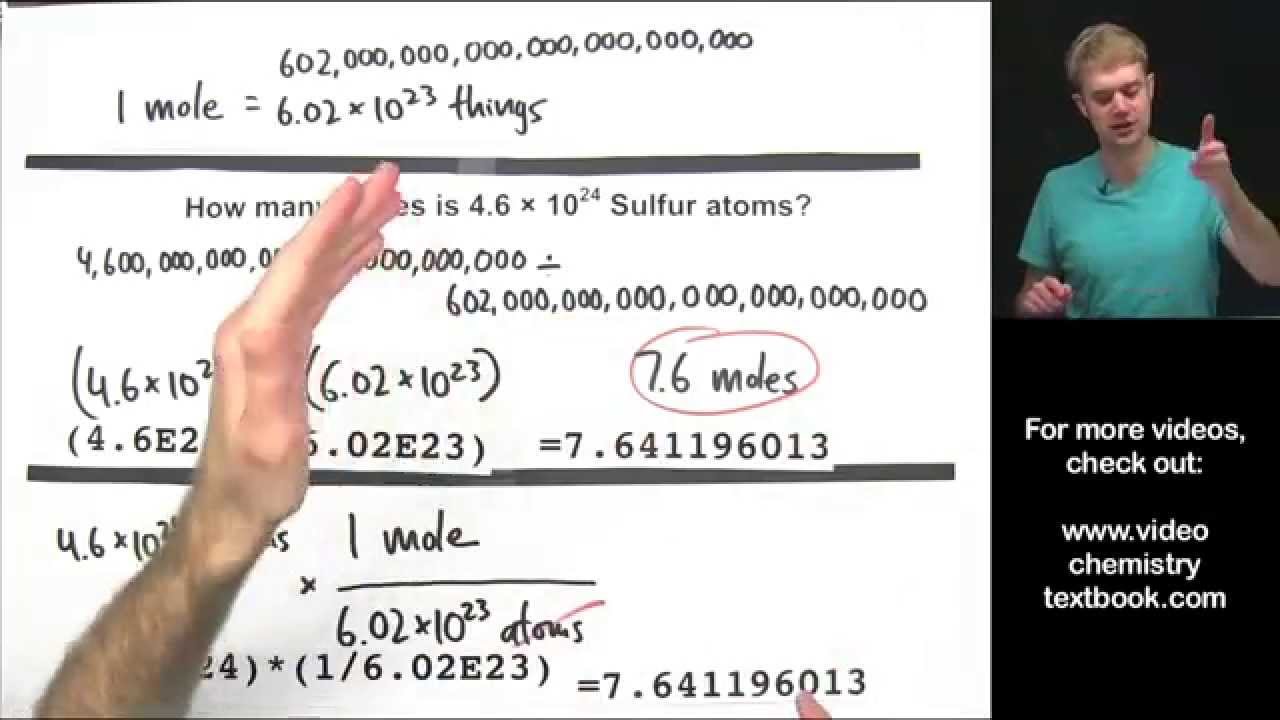
How many molecules are in 0.75 moles of co2?
Answer. =4.5165*10^23 each molecule present in co2 molecule.
How many molecules are in 0.75 moles of oxygen gas?
∴ 0.75 moles have 0.75×6.023×10²³ molecules.
How do you find the number of molecules?
Explanation: Determine the mass of the substance, and its molar mass. Divide the given mass by its molar mass to get moles, then multiply times 6.022×1023molecules1mol .
How many atoms are present in HCl?
Two atomsEvery HCl molecule contains two atoms, H and Cl.
How many grams are there in 2.0 moles of HCl?
2 moles HCl to grams = 72.92188 grams.
How many molecules are in 2.5 moles of o2?
Expert-verified answer
Therefore, a number of atoms present in 2.50 moles of oxygen are 3.01 ×10^24.
What is the number of moles in 36.5 gram of hydrochloric acid?
The molar mass of HCl is 36.5 g/mol. When we divide mass with atomic/molar mass, we get number of moles. =0.994 moles.
How many grams are in one mole of HCl?
The mass of one mole of any substance is its molar mass, so the mass of one mole of HCl = 1 + 35.5 = 36.5g.
How many grams is a molecule of hydrochloric acid?
| Reagent | HCl | HNO3 |
|---|---|---|
| Density (g/mL) | 1.18 | 1.41 |
| % Acid or base (by mass) | 37.3 | 70.0 |
| Molecular weight (g/mol) | 36.47 | 63.02 |
| Molarity of conc. acid or base (mol/L) | 12 | 16 |
How many molecules are there in 5h2o?
Answer: 1. So here you have five molecules of water with two hydrogen atoms and one oxygen atom per molecule.
Molar Mass / Molecular Weight of HCl : Hydrochloric acid
Images related to the topicMolar Mass / Molecular Weight of HCl : Hydrochloric acid
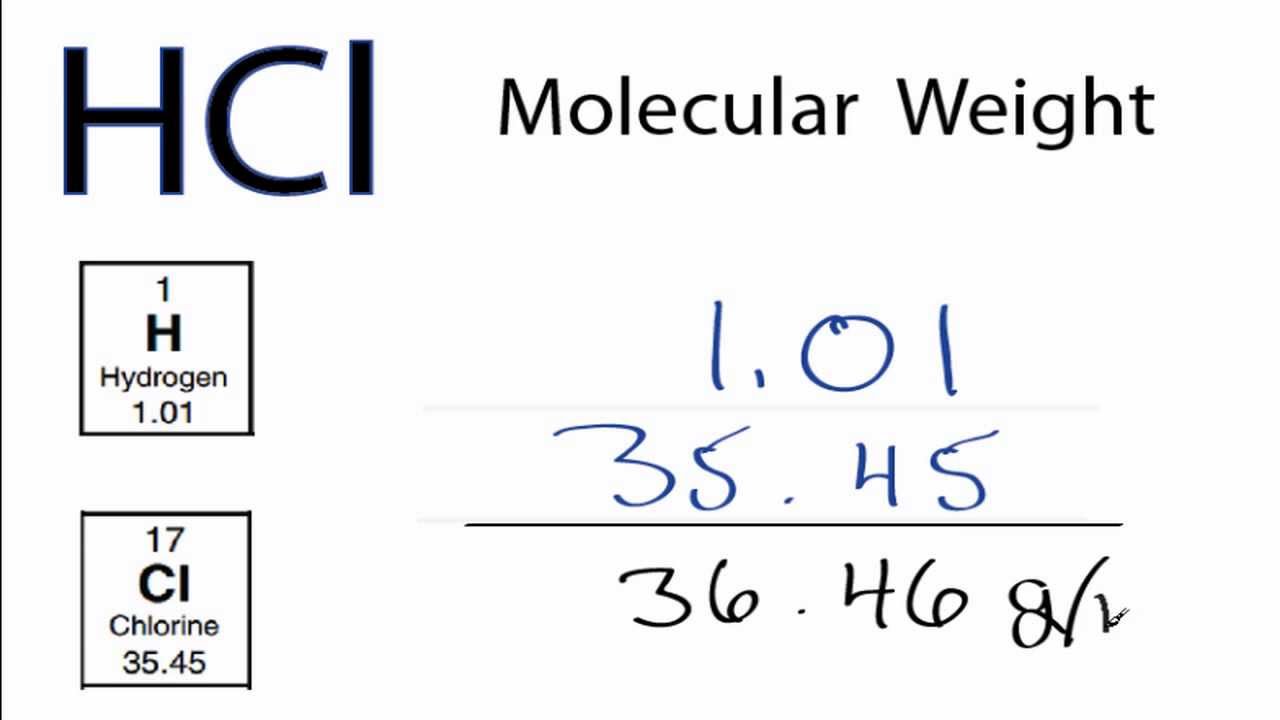
How many atoms are in 0.35 moles?
0.35 mole = 2.1077 × 10^23 carbon atoms.
How many molecules are in 4 mol mol of molecules?
The answer is 1.660538863127E-24. We assume you are converting between mole and molecule. You can view more details on each measurement unit: moles or molecule The SI base unit for amount of substance is the mole. 1 mole is equal to 6.0221415E+23 molecule.
Related searches
- how is the mole similar to a dozen
- how to calculate number of moles of hcl
- how many atoms are in 2 00 moles of li
- which weighs more atoms a mole of copper or a mole of gold
- how many atoms are in 55g of iron
- how many atoms are in 3p2o5?
- how many atoms are in 3p2o5
- what is the mass of 3 13×1021 particles of kbr
- how many atoms are in 2.00 moles of li
- how many atoms are in 55g of iron?
- how many atoms are in 55 grams of iron
- how many moles are in 6.02 x 1023 atoms of carbon?
- how many molecules are in 0.83 moles of hcl
- how many moles are in 6 02 x 1023 atoms of carbon
- if you have 0 00812 mole of h2co3 how many molecules do you have
Information related to the topic how many molecules are in 0.83 moles of hcl
Here are the search results of the thread how many molecules are in 0.83 moles of hcl from Bing. You can read more if you want.
You have just come across an article on the topic how many molecules are in 0.83 moles of hcl. If you found this article useful, please share it. Thank you very much.

