Let’s discuss the question: how many molecules are in a mole of caffeine. We summarize all relevant answers in section Q&A of website Myyachtguardian.com in category: Blog MMO. See more related questions in the comments below.
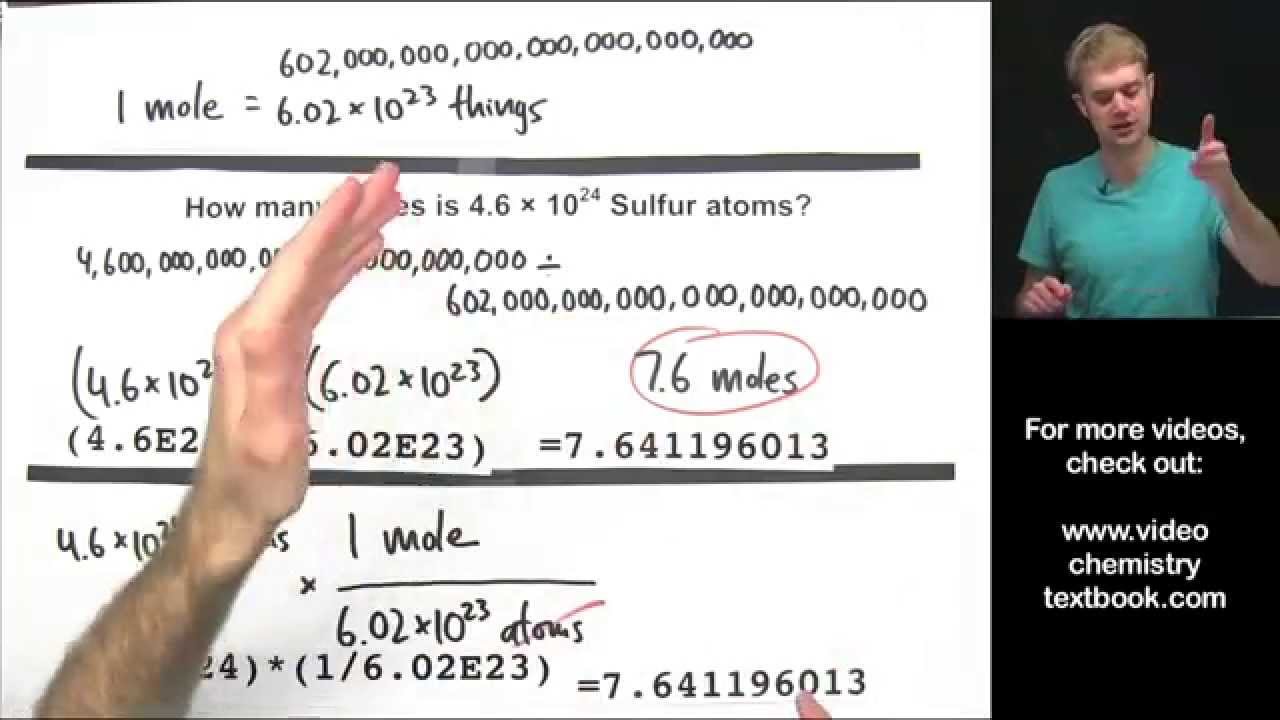
Table of Contents
How many moles are in caffeine?
The molecular mass of caffeine is 194.19 g⋅mol−1 . Moles of caffeine in a cup = 125×10−3g194.19⋅g⋅mol−1 = ?? mol .
How many molecule are in a mole?
The mole is represented by Avogadro’s number, which is 6.022 × 1023 atoms or molecules per mol.
Converting Between Moles, Atoms, and Molecules
Images related to the topicConverting Between Moles, Atoms, and Molecules

What is the mass of 1 mole of caffeine?
How many molecules of caffeine are in coffee?
An average cup of coffee contains about 125 mg of caffeine, C8H10N4O2.
What is a caffeine molecule?
Caffeine is a trimethylxanthine in which the three methyl groups are located at positions 1, 3, and 7. A purine alkaloid that occurs naturally in tea and coffee. It has a role as a central nervous system stimulant, an EC 3.1.
How many molecules are in 2 moles?
If we have 2 mol of Na atoms, we have 2 × (6.022 × 10 23) Na atoms, or 1.2044 × 10 24 Na atoms. Similarly, if we have 0.5 mol of benzene (C 6H 6) molecules, we have 0.5 × (6.022 × 10 23) C 6H 6 molecules, or 3.011 × 10 23 C 6H 6 molecules.
Is mole a molecule?
Although the two terms moles and molecules are distinct terms, the concept of moles can be used to measure the amount of molecules present in a sample. The main difference between mole and molecule is that mole is a unit of measurement of quantity whereas molecule is a chemical species that is made out of atoms.
How many molecules is 3 moles?
A mole of anything contains 6.022×1023 individual items of that something. You have 3 moles, so there are 3×6.022×1023 oxygen molecules .
How do you convert moles to molecules?
- To go from moles to molecules, multiply the number of moles by 6.02 x 1023.
- To go from molecules to moles, divide the numbers of molecules by 6.02 x 1023.
How many atoms are in a molecule of caffeine?
The chemical composition for caffeine is C8H10N4O2 which means it has 8 carbon atoms, 10 hydrogen atoms, 4 nitrogen atoms, and 2 oxygen atoms.
How many moles of carbon are in 2.0 moles of caffeine?
3. There are 16 mol of carbon in 2.0 mol of caffeine.
Avogadro’s Number, The Mole, Grams, Atoms, Molar Mass Calculations – Introduction
Images related to the topicAvogadro’s Number, The Mole, Grams, Atoms, Molar Mass Calculations – Introduction

How do I calculate moles?
- Measure the weight of your substance.
- Use a periodic table to find its atomic or molecular mass.
- Divide the weight by the atomic or molecular mass.
- Check your results with Omni Calculator.
What is the molecular formula for coffee?
| PubChem CID | 6850756 |
|---|---|
| Molecular Formula | C25H28N6O7 |
| Synonyms | COFFEE EXTRACT 84650-00-0 furan;1-methylpyridin-1-ium-3-carboxylate;pyridine-3-carboxylic acid;1,3,7-trimethyl-4,5-dihydropurine-2,6-dione Coffee, Coffea arabica, ext. |
| Molecular Weight | 524.5 |
How many moles of caffeine are in a shot of espresso?
Espresso There is 1.00×102mg of caffeine in a shot of. espresso.
What macromolecule is caffeine?
…
Caffeine.
| Clinical data | |
|---|---|
| Protein binding | 25–36% |
| Metabolism | Primary: CYP1A2 Minor: CYP2E1, CYP3A4, CYP2C8, CYP2C9 |
Can you be addicted to caffeine?
People can develop a dependence on coffee and other Caffeinated beverages quite quickly. This is due to the chemical changes that sustained consumption produces in the brain. If someone drinks Caffeine on a daily basis, they will develop a tolerance just as they would to other drugs or alcohol.
Is caffeine a molecular compound?
How is caffeine metabolised?
Caffeine is almost completely metabolized with 3% or less being excreted unchanged in urine [3,6]. The main route of metabolism in humans (70–80%) is through N-3-demethylation to paraxanthine also known as 1,7-dimethylxanthine or 17X [3,6,7] (see Fig. 1). This reaction is carried out by CYP1A2 in the liver [6].
What class of molecule is caffeine?
Caffeine, also known as coffein or mateina, belongs to the class of organic compounds known as xanthines.
How many molecules are in 1.5 moles?
Hence, number of molecules in 1.5 moles of ammonia is 9.033 × 1023.
Concept of Mole – Part 1 | Atoms and Molecules | Don’t Memorise
Images related to the topicConcept of Mole – Part 1 | Atoms and Molecules | Don’t Memorise

How many molecules are there in 1 mole of CO2?
Mass of 1 mole (6.023 X 1023 molecules) of CO2 is about 44g.
How many molecules of water are in 5 moles?
We know that 1 mol water contains 6.023×10^23 molecules . Hence , 5 mol water contain 5×6.023×10^23 = 3.0115 × 10^24 molecules.
Related searches
- how many molecules are in caffeine
- how much does a mole of diatomic nitrogen n2n2 weigh
- in 50 0 grams of caffeine how many caffeine molecules are present
- caffeine molecular formula
- caffeine molecule
- how many moles of caffeine are in a cup
- molar mass of caffeine
- how many moles of caffeine c8h10o2n4 are contained in a 100 mg sample of caffeine
- how many grams of caffeine c8h10n4o2 contain 2 53 x10 24 molecules of caffeine
- in 50.0 grams of caffeine how many caffeine molecules are present
- how many moles in caffeine
- how many moles of caffeine c8h10o2n4 are contained in a 100. mg sample of caffeine
Information related to the topic how many molecules are in a mole of caffeine
Here are the search results of the thread how many molecules are in a mole of caffeine from Bing. You can read more if you want.
You have just come across an article on the topic how many molecules are in a mole of caffeine. If you found this article useful, please share it. Thank you very much.

