Let’s discuss the question: how many moles of silver atoms are in 1.8. We summarize all relevant answers in section Q&A of website Myyachtguardian.com in category: Blog MMO. See more related questions in the comments below.

Table of Contents
How many atoms are in a mole of silver?
From Avogadro’s number, we know that one mole of silver atoms is equal to 6.02 times 10 to the 23rd silver atoms.
How do you find moles of silver atoms?
The molar mass of silver is 107.9 g/mol. Therefore: n moles of silver atoms will have a mass of (n TIMES 107.9) g. The problem says: 6.89 g EQUALS (n TIMES 107.9) g; so we solve for n by dividing 6.89 by the molar mass (107.9). This (n) gives us the mole count.
Moles To Atoms Conversion – Chemistry
Images related to the topicMoles To Atoms Conversion – Chemistry

How many atoms of silver are in 2 moles?
And, thus in 2.00⋅mol of silver, there are 2⋅mol×NA = 6.022×1023⋅mol−1 ×2⋅mol =1.20×1024 individual silver atoms. What is the mass of this number, this quantity of silver atoms?
What is the mass of 1 mole silver?
Grade 10. i) The molar mass of silver is 107.9 g/mol. (rounding to 108). As 1 mole = 6.023 x 10^23 molecules (Avogadro’s number), 108 g of Silver contains 6.023 x 10^23 molecules.
How many atoms are there in 1g of silver?
Perhaps you mean 1 gram/mol the number of silver atoms in one gram of silver. This number gmol is expressed by the Avogadro constant , which has a value of approximately 6.022140857×10^23 atoms/mol. The mole is an SI base unit , with the unit symbol mol.
What are silver atoms?
Silver is the second element in the eleventh column of the periodic table. It is classified as a transitional metal. Silver atoms have 47 electrons and 47 protons with 60 neutrons in the most abundant isotope.
How many moles are there in 100g of silver?
›› More information from the unit converter
The answer is 107.8682.
How many silver atoms are there in 3.75 moles of silver?
Answer and Explanation: 3.75 moles of silver (Ag) contains 2.26 x 1024 Ag atoms.
How do I calculate moles?
- Measure the weight of your substance.
- Use a periodic table to find its atomic or molecular mass.
- Divide the weight by the atomic or molecular mass.
- Check your results with Omni Calculator.
How many atoms are there in 12g of magnesium?
There are 1 mole (6.02 x 10^23) of Mg atoms in 24g of Mg. So, in 12g of Mg, there is 0.5 moles of Mg present, which translates to roughly 3.01 x 10^23 atoms.
How many atoms are there in exactly 12g of carbon-12 element?
(a) 6.022 × 1023 atoms are present in 12g of carbon-12 element.
Converting Between Moles, Atoms, and Molecules
Images related to the topicConverting Between Moles, Atoms, and Molecules
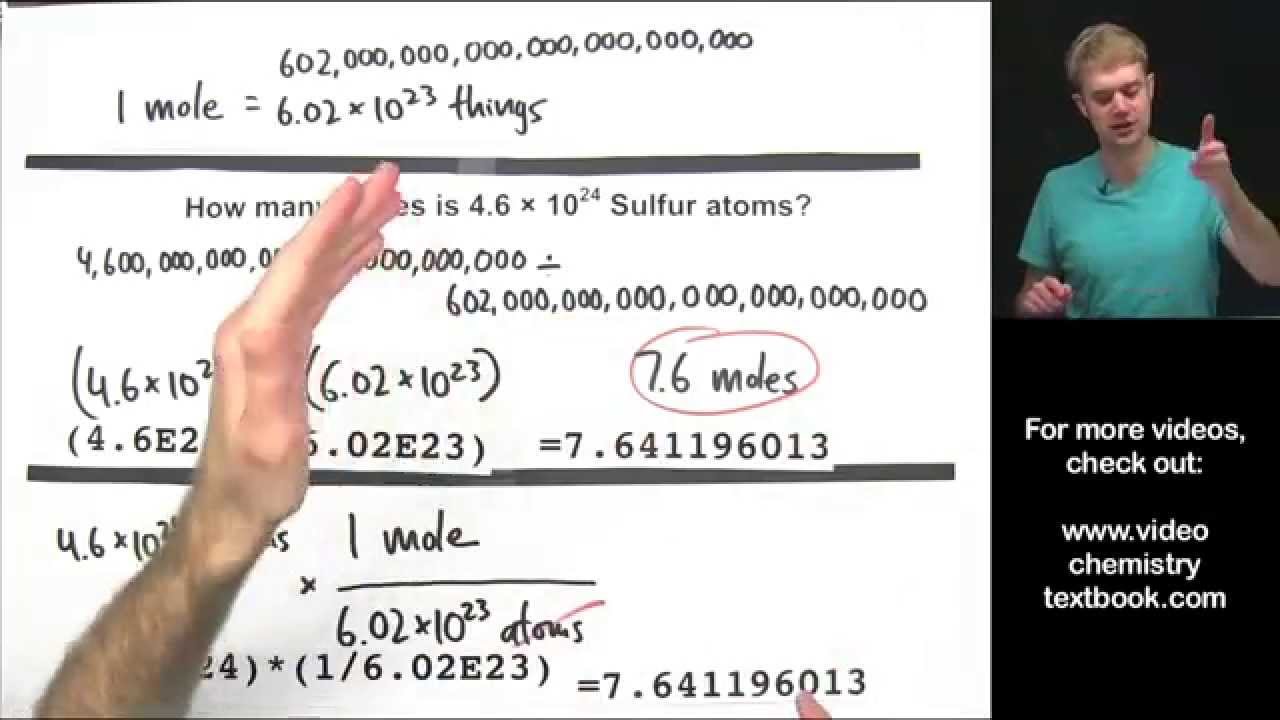
How many water molecules are there in 2.0 moles of water h2o )?
One mole of H₂O contains 6.023 × 10²³ number of H₂O molecules. This is so because 1 mole of any substance contains the amount of 6.023 × 10²³ constituents in it, also known as the Avogadro number. Thus, 2 moles of H₂O will contain 2 × 6.023 × 10²³ number of H₂O molecules, that is 12.046 × 10²³ molecules of H₂O.
What is the mass of 1.75 moles of silver?
Furthermore, the atomic mass of silver is 107.868. That means that one mole of silver weighs 107.868 grams (107.868 g/mol). Based on that information, to convert 1.75 moles of silver to grams, we multiply 1.75 moles of silver by 107.868.
How many grams are there in 2.3 moles of silver?
Answer: The mass of 2.3 × 10²⁴ Ag atoms is approximately 410 g.
What is the mass of 0.5 moles of silver?
The mass of 0.5 mole of silver is equal to 54g.
The number of the moles is equivalent to the given mass which is then divided by the total molar mass.
How do you convert moles to atoms?
Avogadro’s number is a very important relationship to remember: 1 mole = 6.022×1023 6.022 × 10 23 atoms, molecules, protons, etc. To convert from moles to atoms, multiply the molar amount by Avogadro’s number. To convert from atoms to moles, divide the atom amount by Avogadro’s number (or multiply by its reciprocal).
What is the mass of 1 gram atom of silver?
Similarly one gram atom of silver = gram atomic weight of silver = 107.8 g (atomic mass of silver = 107.8 u) .
How many atoms are there in 2.00 moles of sodium?
There are 1.20⋅1024 atoms of sodium in 2 moles of sodium.
What element is Z 18?
Argon is it is colorless, tasteless and odorless noble gas that is located in Group 18 on the Periodic Table. It was discovered by Henry Cavendish in 1785 and was named Argon, which is derived from the Greek word “argos” meaning inactive.
What is silver’s atomic number?
How do you convert 12g of oxygen gas to moles?
- Given mass of O 2 = m = 12g.
- Molar Mass of O 2 = M = 32g.
- Given mass of H 2 O = m = 20g.
- Molar Mass of H 2 O = M = 18g.
Avogadro’s Number, The Mole, Grams, Atoms, Molar Mass Calculations – Introduction
Images related to the topicAvogadro’s Number, The Mole, Grams, Atoms, Molar Mass Calculations – Introduction

Which has more no of atoms 100g of silver in 100g of iron?
Answer: 100g of silver has more atoms than 100g of iron.
How do you convert moles to grams formula?
Multiply the given number of moles (2.50 mol) by the molar mass (122.548 g/mol) to get the grams.
Related searches
Information related to the topic how many moles of silver atoms are in 1.8
Here are the search results of the thread how many moles of silver atoms are in 1.8 from Bing. You can read more if you want.
You have just come across an article on the topic how many moles of silver atoms are in 1.8. If you found this article useful, please share it. Thank you very much.

