Let’s discuss the question: how many nonpolar bonds does ch3oh contain. We summarize all relevant answers in section Q&A of website Myyachtguardian.com in category: Blog MMO. See more related questions in the comments below.
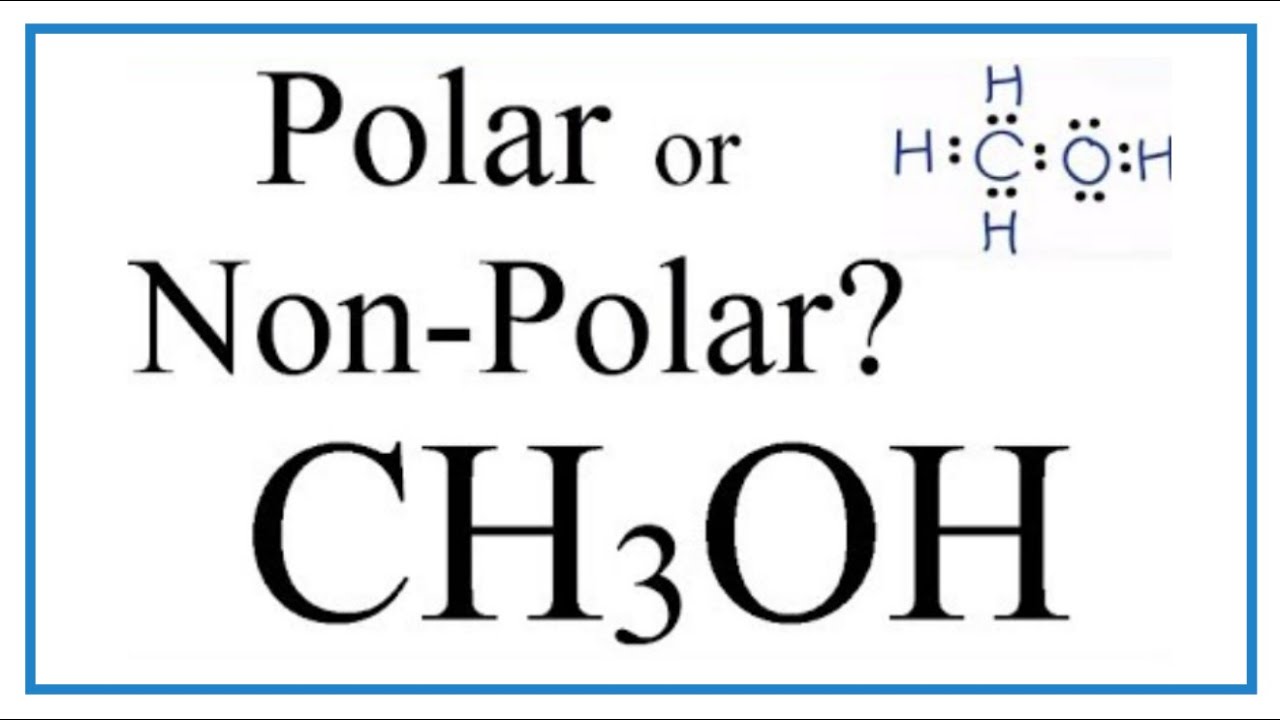
Table of Contents
Does CH3OH have nonpolar bonds?
Yes. To begin with, methanol, H3C−OH , is asymmetrical, so it could not be nonpolar (being 100% symmetrical in all directions means all dipole moments would cancel out completely).
How many bonds does CH3OH have?
To know the kind of hybridization CH3OH has, take a look at the structure of CH3OH. Notice here that the C atom has 4 sigma bonds. We can also say that C has 4 attached atoms (3 Hydrogen, 1 Oxygen). Thus, there is a total of 4 sigma bonds.
Is CH3OH Polar or Nonpolar? (Methanol)
Images related to the topicIs CH3OH Polar or Nonpolar? (Methanol)

What is methanol polar or nonpolar?
Any molecule with lone pairs of electrons around the central atom is polar. Methanol is polar. This is not a symmetric molecule. The −OH side is different from the other 3 −H sides.
Is tetrahedral CH3OH polar or nonpolar?
CH3OH is a polar molecule as the dipole-dipole moment is not canceled due to its asymmetric shape.
What is the intermolecular forces of ch3oh?
Ch3oh intermolecular forces has hydrogen bonding, dipole dipole attraction and London dispersion forces.
What is the bond angle of ch3oh?
The CH3OH bond angle will be about 109 degrees since it has a tetrahedral molecular geometry.
How many sigma and pi bonds are in ch3oh?
The number of ‘sigma’ and π electrons in methanol are
Methanal(H-H∣C=O) contains 3 σ bonds ∴6electrons and one π bond ∴ 2 electrons.
Polar and NonPolar Molecules: How To Tell If a Molecule is Polar or Nonpolar
Images related to the topicPolar and NonPolar Molecules: How To Tell If a Molecule is Polar or Nonpolar

How many sigma and pi bonds are present in ch3oh?
There are 5 sigma bonds and 0 pi bonds in this molecule.
What type of compound is methanol ch3oh?
It is an alkyl alcohol, a one-carbon compound, a volatile organic compound and a primary alcohol. It is a conjugate acid of a methoxide.
Is n2 polar or nonpolar?
Answer: N2 is a nonpolar. In an N2 molecule, bonds are formed between two nitrogen atoms. As both are identical atoms they have the same value of electronegativity so there is no difference between electronegativity and the net dipole moment becomes zero, Hence N2 is a nonpolar molecule.
What intermolecular forces are in N2?
Frequently Asked Questions on N2 Intermolecular Forces
Since N2 molecules are nonpolar, only dispersion forces exist.
What is the strongest intermolecular force in a sample of CH3OH?
The strongest intermolecular forces in methanol are hydrogen bonds. This compound is also known to feature relatively strong dipole-dipole interactions.
What is the IMF for i2?
Molecular iodine is completely non-polar. As such, the only intermolecular force between molecules of iodine is the London dispersion force.
The Chemical Bond: Covalent vs. Ionic and Polar vs. Nonpolar
Images related to the topicThe Chemical Bond: Covalent vs. Ionic and Polar vs. Nonpolar

How many electrons does CH3OH have?
The molecule methanol (methyl alcohol) has the structure CH3OH, and contains fourteen valence electrons (four for carbon, six for oxygen, one each for the four hydrogens).
What is the elements of CH3OH?
Methanol is a liquid chemical with the formula CH3OH (often abbreviated MeOH). It is colorless, volatile, flammable, and poisonous. Methanol is made from the destructive distillation of wood and is chiefly synthesized from carbon monoxide and hydrogen.
Related searches
- what type of bond will typically form between boron and hydrogen based on their electronegativity
- is asbr5 polar or nonpolar
- how many polar bonds does ch4 contain?
- which compound has only ionic bonds?
- what is the molecular shape and polarity for xenon tetrafluoride quizlet
- according to formal charge which one is the most stable structure
- using vsepr theory, predict the molecular shape and bond angles in bcl3.
- how many polar bonds does ccl4 have in its structure
- how many polar bonds does ch4 contain
- how many bonds does c have in ch3oh
- in the following molecular shape the bond angles are
- does ch3oh have polar bonds
- using vsepr theory predict the molecular shape and bond angles in bcl3
- use vsepr theory to predict the molecular geometry of if5
- how many polar bonds does ccl4 have in its structure?
- how many bonds does ch3oh have
Information related to the topic how many nonpolar bonds does ch3oh contain
Here are the search results of the thread how many nonpolar bonds does ch3oh contain from Bing. You can read more if you want.
You have just come across an article on the topic how many nonpolar bonds does ch3oh contain. If you found this article useful, please share it. Thank you very much.

