Let’s discuss the question: how many of the following molecules possess dipole moments. We summarize all relevant answers in section Q&A of website Myyachtguardian.com in category: Blog MMO. See more related questions in the comments below.
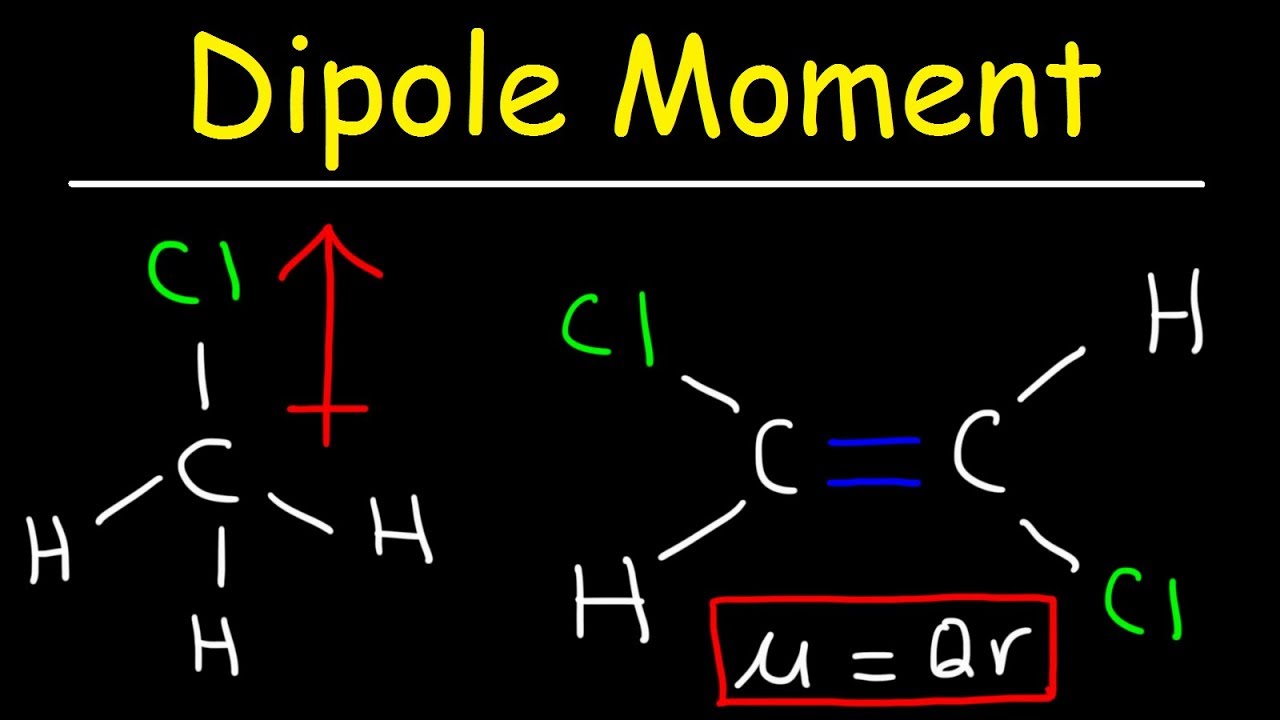
Table of Contents
What molecules possess a dipole moment?
- carbon dioxide: 0 (despite having two polar C=O. …
- carbon monoxide: 0.112 D.
- ozone: 0.53 D.
- phosgene: 1.17 D.
- water vapor: 1.85 D.
- hydrogen cyanide: 2.98 D.
- cyanamide: 4.27 D.
- potassium bromide: 10.41 D.
Does bh3 possess dipole moments?
The bonds in BH3 will therefore be somewhat polarized, with the local dipoles oriented towards the hydrogen atoms, as shown below. But because the molecule is symmetrical, the three dipole arrows cancel and, as a molecule, BH3 has no net molecular dipole.
Dipole Moment, Molecular Polarity \u0026 Percent Ionic Character
Images related to the topicDipole Moment, Molecular Polarity \u0026 Percent Ionic Character

Does ccl4 have a dipole moment?
– The polar molecule results from the unequal sharing of electrons which are the valence electrons and in the molecules like carbon tetrachloride these bonds are evenly distributed and cancel out. Thus there is no net dipole moment and the compound is non polar in nature.
Does CH3 2O have a net dipole moment?
Yes, (CH3)2O ( C H 3 ) 2 O has a dipole moment. There is enough difference in electronegativity between oxygen and carbon (net 1.0) In looking at… See full answer below.
Which one of the following molecules has a dipole moment of zero?
All the three molecules methane, carbon tetrabromide and acetylene have zero dipole moments as the individual bond dipoles cancel each other which result in zero net dipole moments.
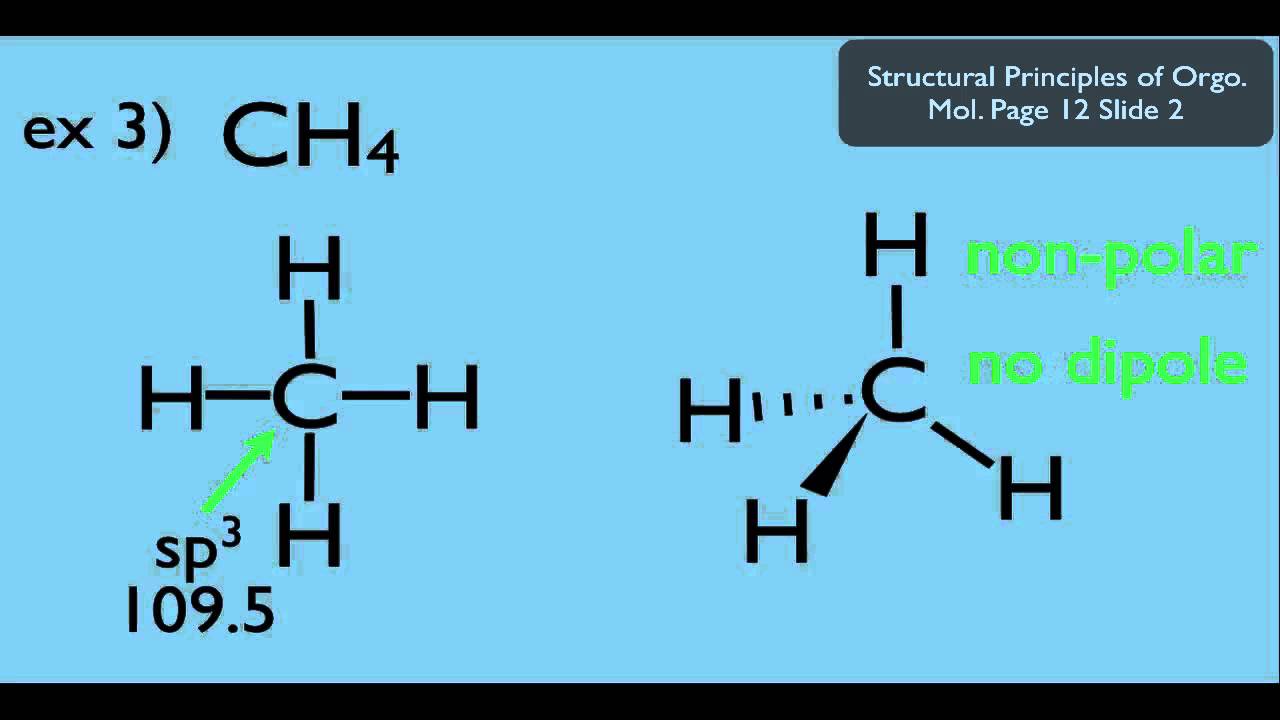
Does CH4 have a dipole moment?
The dipole moment of methane CH4 is zero.
Does no2 have a dipole moment?
Nitrite ions exist in bent shape, in which the bonds are not exactly opposite. Thus, the molecule has a dipole moment.
How many bonds have a dipole in NH3?
Again, NH3 is a polar molecule because it has three dipoles, and these dipoles do not cancel out each other, plus they have net dipole moments.
Does PH3 have a dipole moment?
PH3 is called phosphine and it is quite toxic and flammable. PH3 must be polar since it is not symmetrical. PH3 has a lone pair and does not have a trigonal planar geometry–for this reason it is not symmetrical. The dipole moment of phosphine is 0.58D which is less than 1.42D for NH3.
Is H2 dipole-dipole?
If the molecules have no dipole moment, (e.g., H2, noble gases etc.) then the only interaction between them will be the weak London dispersion (induced dipole) force.
Does sh2 have a dipole moment?
Hydrogen sulfide is slightly polar because of the presence of lone pair of electrons in Sulfur and the electronegativity difference between Sulfur and H atoms. There are eight valence electrons present in the molecule of hydrogen sulfide. Hydrogen sulfide molecule has an angular geometry with a non-zero dipole moment.
How to Determine Whether a Molecule has an Overall Molecular Dipole Moment
Images related to the topicHow to Determine Whether a Molecule has an Overall Molecular Dipole Moment

What is dipole moment class 11th?
A dipole moment is the product of the magnitude of the charge and the distance between the centers of the positive and negative charges. It is denoted by the Greek letter ‘µ’. Mathematically, Dipole Moment (µ) = Charge (Q) * distance of separation (r) It is measured in Debye units denoted by ‘D’.
What is dipole Class 11?
Hint: A dipole is a pair of charges which are equal in magnitude and opposite in charge that are separated by a distance. Dipoles are present in molecules when there is a separation of charges or we can say, the constituent atoms possess an electric charge.
What is dipole moment chemistry class 11?
Dipole moment is the product of electric charge and distance between the positive and negative species present in the molecule.
Does diphenyl ether have a dipole moment?
Abstract. The presence of a large molecular dipole moment in diphenyl ethers leads unequivocally to a centrosymmetric crystal structure.
What is the dipole moment of CH3?
CH3 CH3 (Dipole moment =u) CH3 Dipole moment = 2.
What is the dipole moment of CHCl3?
According to the 55’th edition of the Handbook of Chemistry and Physics the dipole moment of dichoromethane , CH2Cl2, is 1.6 debye and the dipole moment of chloroform , CHCl3, is 1.01 debye.
Which of the following pairs both molecules possess dipole moment?
H2OandSO2 both possess dipole moment due to bent structure.
Which one of the following has the largest dipole moment?
Thus , CH2Cl2 has highest dipole moment.
What is the dipole moment of HBr?
The dipole moment of HBr is 2.60D and the interatomic spacing is 1.41 pm.
The Dipole Moments of Molecules
Images related to the topicThe Dipole Moments of Molecules
Does IF7 have a dipole moment?
Answer: IF7 are nonpolar (their shape is complex, but the dipole moment is zero or close to it).
Does SnCl2 have a dipole moment?
BeCl2 has zero dipole moment while SnCl2 has a dipole moment.
Related searches
- which of the following molecules has a permanent dipole moment
- what is the dipole moment of a molecule
- which of the following molecules have a dipole moment
- how to tell if molecule has dipole moment
- which of the following molecules has a net dipole moment
- which one of the following molecules has a dipole moment
- which of the following molecules has no dipole moment?
- how to find dipole moment of a molecule
- which of the following molecules has no dipole moment
- how many of the following molecules or ions contain double or triple bonds
- which of the following molecules does not have a dipole moment
- how many bonds have a dipole in so2
- what molecules have dipole moments
Information related to the topic how many of the following molecules possess dipole moments
Here are the search results of the thread how many of the following molecules possess dipole moments from Bing. You can read more if you want.
You have just come across an article on the topic how many of the following molecules possess dipole moments. If you found this article useful, please share it. Thank you very much.

