Let’s discuss the question: how to convert dalton to gram. We summarize all relevant answers in section Q&A of website Myyachtguardian.com in category: Blog MMO. See more related questions in the comments below.

Table of Contents
How do you convert Daltons to grams per mole?
The atomic mass constant, denoted mu is defined identically, giving mu = m(12C)/12 = 1 Da. A unit dalton is also approximately numerically equal to the molar mass of the same expressed in grams per mole (NA Da ≈ 1 g/mol).
What is the conversion between Daltons and grams?
dalton to Gram conversion
Conversion number between dalton [Da] and Gram [g] is 1.66053904 × 10–24. This means, that dalton is smaller unit than Gram.
Amu to gram and gram to amu conversion
Images related to the topicAmu to gram and gram to amu conversion

How do you convert Daltons to molecular weight?
Measure of molecular weight or molecular mass. One molecular hydrogen molecular atom has molecular mass of 1 Da, so 1 Da = 1 g/mol.
How do you convert Daltons to molarity?
To understand how to do a protein molarity calculation, let’s begin with important conversions. 1 Dalton (Da) = 1 g/mol, this means that 1 KDa = 1000 g/mol = 1 kg/mol. Let’s do a common example, getting the molarity of an antibody. An antibody has a molecular weight of 150kDa or 150000 Da.
Is Dalton the same as g mol?
A unit dalton is also approximately numerically equal to the molar mass of the same expressed in grams per mole (NA Da ≈ 1 g/mol). Prior to the 2019 redefinition of the SI base units these were numerically identical by definition (NA Da = 1 g/mol) and are still treated as such for most purposes.
How many amu are in a Dalton?
The dalton (symbol: Da), also known as an atomic mass unit, is a unit of mass that is equal to one twelfth of the mass of a free carbon-12 atom at rest. Its value is approximately equal to 1.660 x 10−27 kg.
What is a dalton measurement?
The dalton (symbol: Da), also known as an atomic mass unit, is a unit of mass that is equal to one twelfth of the mass of a free carbon-12 atom at rest. Its value is approximately equal to 1.660 x 10−27 kg.
Is dalton and Amu the same?
The atomic mass unit (amu) was not renamed to dalton (Da). These are different, albeit related, units.
How many moles are in a dalton?
The mole was defined in such a way that the molar mass of a compound, in g/mol, is numerically equal (for all practical purposes) to the average mass of one molecule, in daltons. Thus, for example, the average mass of a molecule of water is about 18.0153 daltons, and the molar mass of water is about 18.0153 g/mol.
How do you convert molecular weight to grams?
In order to convert the moles of a substance to grams, you will need to multiply the mole value of the substance by its molar mass.
What is the molecular weight of this enzyme in dalton?
Catalase is a common enzyme with a molecular weight of 240,000 daltons, which is equivalent to 240,000 g/mol. It is a tetramer consisting of 4 equal subunits with a molecular weight of 60,000 daltons each.
How do I calculate molecular weight?
molecular weight = (number of carbon atoms)(C atomic weight) + (number of H atoms)(H atomic weight) so we calculate as follows: molecular weight = (6 x 12.01) + (14 x 1.01)
10 SG1 #7 Convert mL to g using density
Images related to the topic10 SG1 #7 Convert mL to g using density
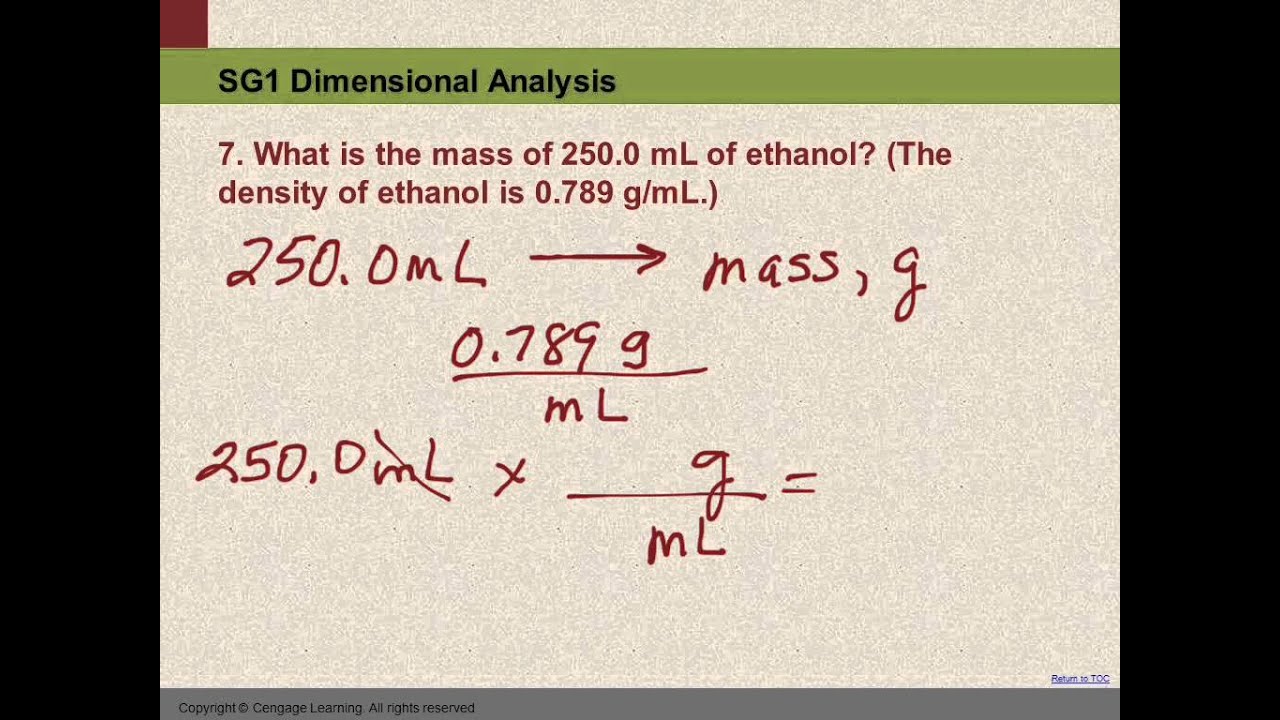
How do you find molarity from g mol?
- First you must calculate the number of moles in this solution, by rearranging the equation. No. Moles (mol) = Molarity (M) x Volume (L) = 0.5 x 2. = 1 mol.
- For NaCl, the molar mass is 58.44 g/mol. Now we can use the rearranged equation. Mass (g) = No. Moles (mol) x Molar Mass (g/mol) = 1 x 58.44. = 58.44 g.
What is g mol means?
The concept that allows us to bridge these two scales is molar mass. Molar mass is defined as the mass in grams of one mole of a substance. The units of molar mass are grams per mole, abbreviated as g/mol.
How do I calculate molarity?
…
To calculate molarity:
- Find the number of moles of solute dissolved in solution,
- Find the volume of solution in liters, and.
- Divide moles solute by liters solution.
Why are proteins measured in daltons?
Protein size is measured in daltons, a measure of molecular weight. One dalton is defined as the mass of a hydrogen atom, which is 1.66 x 10–24 gram. Most proteins have masses on the order of thousands of daltons, so the term kilodalton (kD) is often used to describe protein molecular weight.
How many daltons is an amino acid?
The average molecular weight of an amino acid is 110Da. Dalton (Da) is an alternate name for the atomic mass unit, and kilodalton (kDa) is 1,000 daltons. Thus a protein with a mass of 64kDa has a molecular weight of 64,000 grams per mole.
How did dalton measure atomic mass?
Dalton decided to use hydrogen as the unit for his system of atomic masses. By weight, the ratio of oxygen to hydrogen in water is 7.94:1 and the ratio of nitrogen to hydrogen in ammonia is 4.63:1.
How do you convert Daltons to amu?
…
Dalton to Atomic Mass Unit Conversion Table.
| Dalton | Atomic Mass Unit [u] |
|---|---|
| 0.01 dalton | 0.0099999386 u |
| 0.1 dalton | 0.0999993857 u |
| 1 dalton | 0.9999938574 u |
| 2 dalton | 1.9999877148 u |
What is Dalton in chemistry?
A theory of chemical combination, first stated by John Dalton in 1803. It involves the following postulates: (1) Elements consist of indivisible small particles (atoms). (2) All atoms of the same element are identical; different elements have different types of atom. (3) Atoms can neither be created nor destroyed.
What is Dalton class 9?
Dalton’s atomic theory
Atoms of a specified element are identical in mass and chemical properties. Atoms of different elements have different masses and chemical properties. Atoms combine in the ratio of small whole numbers to form compounds. The relative number and kinds of atoms are constant in a given compound.
Is amu the same as grams per mole?
The mass of a single atom of an element [amu] is numerically equal to the mass [g] of 1 mol of that element, regardless of the element.
Chemistry: Average Atomic Mass (amu, Daltons, etc.) – 2 examples | Homework Tutor
Images related to the topicChemistry: Average Atomic Mass (amu, Daltons, etc.) – 2 examples | Homework Tutor
How do you calculate unified mass?
Find the Atomic Mass of a Given Ratio of Isotopes
Step 1: Multiply the atomic mass of the isotope with its abundance percentage and divide the result by 100. Step 2: Add the values gained from step 1 for each given isotope in the sample.
What is Kilo dalton?
kilodalton (plural kilodaltons) An atomic mass unit equal to 1,000 daltons; usually used to describe the molecular weight of large molecules such as proteins.
Related searches
- convert dalton to g/mol
- dalton to picogram
- what is 1 dalton in grams
- how convert gram into ml
- 1 dalton to gmol
- how many steps are in a gram to molecule conversion
- dalton to kg
- 1 dalton to amu
- 1 dalton to g/mol
- 1 dalton to gram
- 1 dalton to kg
- 1 dalton is equal to how many amu
- how to convert grams into grams
- convert dalton to gmol
Information related to the topic how to convert dalton to gram
Here are the search results of the thread how to convert dalton to gram from Bing. You can read more if you want.
You have just come across an article on the topic how to convert dalton to gram. If you found this article useful, please share it. Thank you very much.

