Let’s discuss the question: how to find maximum number of atoms in a plane. We summarize all relevant answers in section Q&A of website Myyachtguardian.com in category: Blog MMO. See more related questions in the comments below.
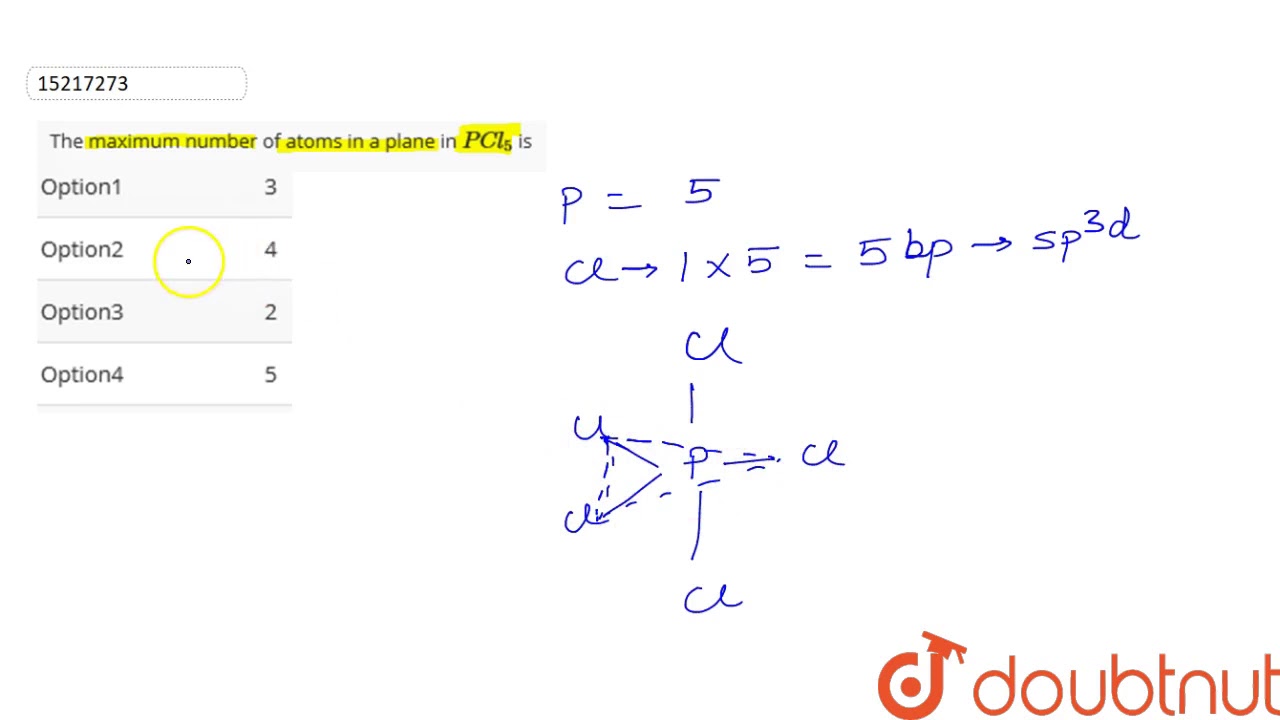
Table of Contents
How do you find the number of atoms in a plane?
For the (100) plane, there are 4 atoms at the 4 corners and one atom in the middle. One fourth of each corner atom is enclosed within the unit cell, and middle atom is entirely within the unit cell, so the number of atoms on the (100) plane within the unit cell is N100 = 4 × (1/4) + 1 × 1 = 2.
What is the maximum number of atoms in the same plane?
Hence, Maximum 4 atoms (one P and 3Cl) are possible in same plane.
The maximum number of atoms in a plane in `PCl_(5)` is
Images related to the topicThe maximum number of atoms in a plane in `PCl_(5)` is

How do you know which has maximum number of atoms?
We can find the elements with the maximum number of atoms can also be found by calculating the number of moles. We know that, number of moles of an element and the number of atoms are directly proportional.
What is the maximum number of atoms lying in one plane of i2cl6?
Maximum number of atom in plane in i2cl6 is 8 and have 3c-4e bonds in structure.
What is maximum number of atoms can lie in the same plane in the molecule PCl3F2?
Three same atoms (chlorine atoms) of PCl3F2 lie in the same plane.
What is maximum number of S atoms in a single plane of s8 molecule?
6. Answer. A. Sulphur has puckered ring (or) crown shape molecule. On same plane S-atoms = 4.
How many atoms are in the same plane in tetrahedral?
Methane has tetrahedral geometry. 3 atoms of methane lie in the same plane.
Which of the following compounds has maximum number of atoms?
Therefore, 18gm CH4 has a maximum number of atoms.
Will the hydrogen atoms be in the same plane or perpendicular planes?
They’re going to be in a plane from front to back. So, in other words, because of the way those pi bonds are orienting themselves, the to the hydrogen are in perpendicular planes.
What is the maximum number of atoms?
Therefore, maximum number of atoms is present in 24 g of C.
Which has maximum number of atoms of oxygen?
Thus there are two moles of atoms present in \[12.044 \times {10^{23}}\] molecules of carbon dioxide. Thus, the maximum number of oxygen atoms are present in \[12.044 \times {10^{23}}\] molecules of carbon dioxide and option (d) is correct.
Which has max no of atoms 24g of C?
UPLOAD PHOTO AND GET THE ANSWER NOW! Step by step solution by experts to help you in doubt clearance & scoring excellent marks in exams. Thus, 24g of C-12 contains maximum number of atoms.
Rank booster: Atoms in the same plane PART 1/2 | NEET | JEE
Images related to the topicRank booster: Atoms in the same plane PART 1/2 | NEET | JEE

What is the maximum number of atoms in b2h6 lie in one plane?
Answer: Four hydrogen atoms, two on the left and two on the right, known as terminal hydrogens and two boron atoms lie in the same plane.
How many atoms lie in the same plane in b2h6?
2 boron atoms and 4 terminal hydrogen atoms lie in the same plane and 4 bridged hydrogens atoms lie in the perpendicular plane.
What is the number of atoms of lye?
Each NaOH has one Na and one O and one H. Therefore, 2 NaOH has 6 atoms.
On which of the following all atoms lie in same plane?
In BF3 B-atom is sp2 hybridised with bond angle 120∘ and is triangular planar in shape So, all atoms lie in one plane .
Why all the atoms involved in resonance must lie in one plane?
All atoms involved in the resonance lie in or near to a single plane, to allow for maximum p-orbital overlap. This constraint is not required of atoms that are not involved in the electron delocalization. 4. All canonical forms must possess the same number of unpaired electrons.
Do all of the atoms in XeF4 lie on a plane?
Answer: hybridization of XeF4 is sp3d2. hence the structure is square bipyramidal. total 5 atoms lie in a plane including Xe.
What is maximum number of S atoms in a single plane of molecule?
Correct answer is ‘7‘.
How many atoms are in 1 mole of sulfur?
One mole of sulfur atoms has a mass of 32.065 g/mol. One mole of sulfur atoms is Avogadro’s number of atoms, which is 6.02 x 1023 atoms.
How do you find the number of atoms in a S8?
256 amu of S8 = 8 moles of S8=8×6.023×10^23 atoms. thank you. Since it is amu, the answer is 8 atoms.
How many atoms are in same plane in methane?
Methane has tetrahedral geometry. 3 atoms of methane lie in the same plane.
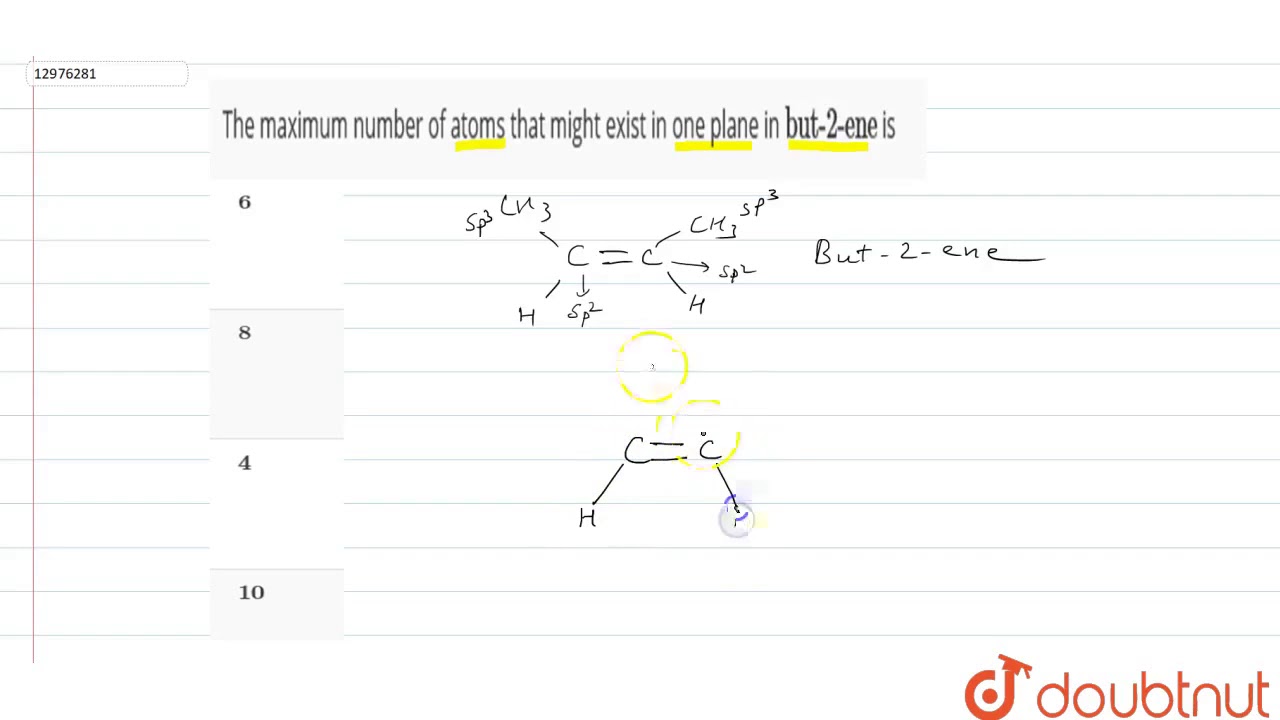
The maximum number of atoms that might exist in one plane in `\”but-2-ene\”` is
Images related to the topicThe maximum number of atoms that might exist in one plane in `\”but-2-ene\”` is
Which structure has maximum same number of identical atoms in same plane?
Phosphorus pentachloride (PCl₅) has trigonal bipyramidal geometry. It has a maximum of four atoms (one Phosphorus and three Chlorine), which are possible in the same plane. Explore more such questions and answers at BYJU’S.
How many atoms of ethene are in the same plane?
In an ethene molecule, the four hydrogen atoms and the two carbon atoms are all in the same plane.
Related searches
- how many of the following molecules have all of their atoms in the same plane?
- the maximum no of s atoms present in a plane in sg molecule
- how do you know if sp2 and sp3 carbons lie in the same plane
- how to find how many atoms in a formula
- maximum number of atom in one plane in nsih33
- how to find maximum number of atoms
- how to determine if atoms are in the same plane
- how many of the following molecules have all of their atoms in the same plane
- maximum number of atoms in a plane
- number of coplanar atoms in b3o6
- maximum number of atom in one plane in n(sih3)3
- carbon atoms in the same plane
- how to find the maximum number of atoms
Information related to the topic how to find maximum number of atoms in a plane
Here are the search results of the thread how to find maximum number of atoms in a plane from Bing. You can read more if you want.
You have just come across an article on the topic how to find maximum number of atoms in a plane. If you found this article useful, please share it. Thank you very much.

