Let’s discuss the question: show the formation of kcl by the transfer of electrons. We summarize all relevant answers in section Q&A of website Myyachtguardian.com in category: Blog MMO. See more related questions in the comments below.

Table of Contents
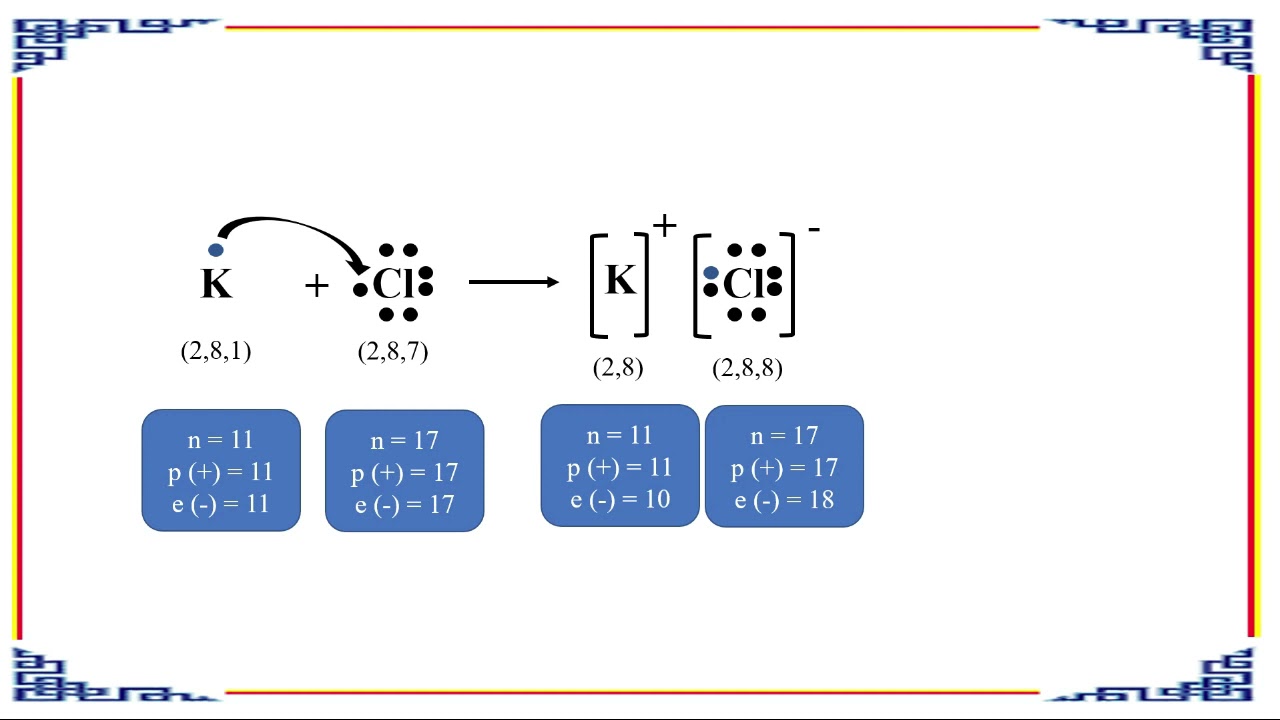
How the formation of KCl by the transfer of electrons?
It consists of a Potassium atom bonding with a Chloride atom. The Potassium chloride chemical formula is KCl. It is formed when the one excess electron in the valence shell of the Potassium atom is transferred to the valence shell of the Chlorine atom so that both the atoms complete their octet valency.
What is the electron configuration of KCl?
Answer: The transfer of electrons showing the formation of KCl can be illustrated as follows in the figure: The “electronic configuration” of “Potassium” (K) is 2,8,8,1 and the “electronic configuration” of “Chlorine” (Cl) is 2,7.
Show the formation of CaS by the transfer of electrons.||covalent and ionic bonds ||electrovalen
Images related to the topicShow the formation of CaS by the transfer of electrons.||covalent and ionic bonds ||electrovalen

How is KCl bond formed?
The oppositely-charged ions formed, K+ and Cl–, are then strongly attracted to each other by strong electrostatic forces in the crystal lattice, called ionic bonds or electrovalent bonds. Hence, the ionic compound potassium chloride with the formula KCl is formed.
What happens to electrons in KCl?
4.2.
This chemical interaction of electrons creates a strong bonding between the atoms as compared to other types of bonds. For example, in the case of Sodium chloride (NaCl) or Potassium chloride (KCl), an electron is transferred between the donor (Na) and acceptor (Cl).
What type of bond does KCl have?
The type of chemical bond that holds together the potassium and chlorine atoms in a potassium chloride molecule is an ionic bond.
Is KCl an ionic or covalent bond?
Yes, KCl, or potassium chloride, is an ionic bond.
Potassium and chlorine are very far apart on the periodic table, with potassium being a metallic…
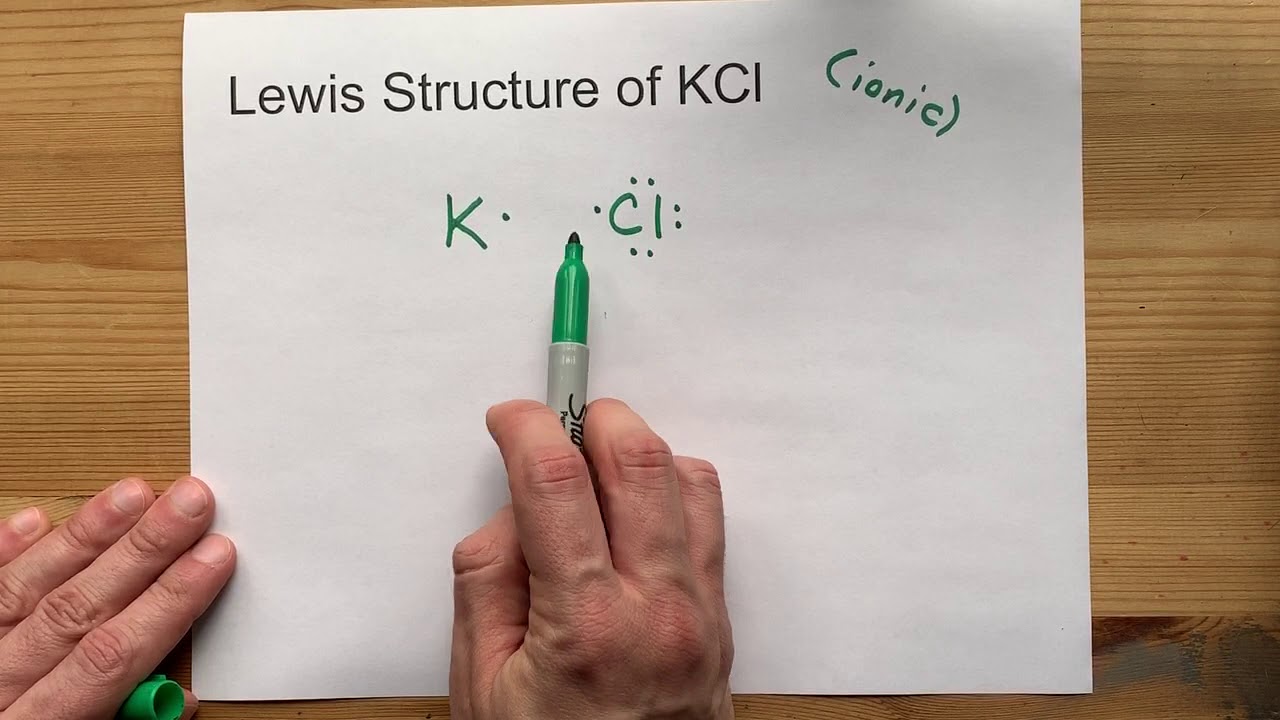
What is the Lewis structure of KCl?
Overview: KCl Lewis Structure
One potassium atom establishes covalent connections with the central chlorine atom as a result, leaving the chlorine atom with three lone pairs. There are three lone pairs of electrons on the chlorine central atom that resists the bond pairs of the K+—-Cl- bond.
Is KCl linear?
The KCl molecule has a linear geometry shape because it contains one potassium atom in the linear and four corners with four lone pairs of electrons. There is one K-Cl single bond at the KCl molecular geometry.
What is the Lewis structure of N2?
…
N2 Lewis Structure| Hybridization & Molecular Geometry.
| Name of molecule | Nitrogen |
|---|---|
| N2 valence electrons | 10 |
What is enthalpy of formation of KCl?
The enthalpy of formation of KCl(s) is -436.7 kJ/mol.
Why does KCl form an ionic bond while cl2 forms a covalent bond?
Now, when potassium reacts with chlorine, the former loses its valence electron and the latter takes it. The two resulting ions, i.e. the potassium cation and the chloride anion, are then bonded together by the electrostatic force of attraction → an ionic bond is formed.
Formation of KCl and Li2O
Images related to the topicFormation of KCl and Li2O
What type of chemical bond is ch3ch2oh?
The C-O and O-H bonds are polar covalent since O is significantly more electronegative than either C or H. Hence there is a slight + charge (d+) on the C and H attached to the O and a slight – charge (d-) on the O. The formal charge on each atom in ethanol is zero.
How many valence electrons are transferred from Calcium to iodine?
Therefore, the calcium atom transfers two valence electrons, one to each iodine atom, to form the ionic bond.
When Calcium forms a bond what will occur?
Q. When Calcium forms a bond, what will occur? Calcium with give away it two valence electrons to form an covalent bond. Calcium will share its two valence electrons to form an ionic bond.
What is the compound name for KCl?
Potassium chloride, KCl, is a naturally occurring potassium salt that, aside from its use as fertilizer, is also a raw material for the production of other important potassium compounds.
Why does KCl not form covalent bonds?
Potassium (K) has atomic number 19 and chlorine (Cl ) has atomic number 17. So, there is deficiency of one electron in Cl atom to complete its octet. Thus, K will transfer its one electron to the Cl atom. Thus, KCl will be an ionic compound as the bond formation is due to the transfer of electron.
Is MgSO4 ionic or covalent?
Structure of MgSO4 Molecules
An ionic bond is formed between the magnesium cation and the sulfate anion in magnesium sulfate.
Does KCl contain covalent bonds?
Answer: (2)
Compound having no covalent bonds is KCl only.
Is KCl s molecular ionic or atomic?
It is an ionic substance in solid state.
What type of structure is exhibited by KCl describe its crystal structure in detail?
KCl Substrate
KCl exhibits a face centered cubic crystal structure with a lattice constant of 6.36 Å.
Draw the Lewis Structure of KCl (potassium chloride)
Images related to the topicDraw the Lewis Structure of KCl (potassium chloride)
What is HCN Lewis structure?
In HCN lewis structure, carbon forms one single bond with the hydrogen atom and a triple bond with the nitrogen atom. The bond angle is 180 degrees and there are 10 valence electrons. HCN is a polar molecule with linear geometry. Exposure to Hydrogen cyanide can be dangerous.
How does the written Lewis structure for KCl differ from HCL?
The Lewis structure of both the compounds is similar. The only difference is that the K – Cl bond involves the complete transfer of electrons, whereas the H – Cl bond involves the sharing of electrons.
Related searches
- formation of kcl equation
- formation of potassium chloride
- in kcl how are the valence electrons distributed
- in kci how are the valence electrons distributed
- kcl electronic configuration
- write the lewis structure for the ionic compound potassium chloride
- kcl molecular structure
- how does the transfer of electrons occur
- how to calculate electrons transferred
- how kcl is formed
- (b) show the formation of kcl by the transfer of electrons
- formation of mgo
- potassium chloride ionic bond diagram
- kcl valency
Information related to the topic show the formation of kcl by the transfer of electrons
Here are the search results of the thread show the formation of kcl by the transfer of electrons from Bing. You can read more if you want.
You have just come across an article on the topic show the formation of kcl by the transfer of electrons. If you found this article useful, please share it. Thank you very much.

