Let’s discuss the question: what is the molecular formula for the molecule shown. We summarize all relevant answers in section Q&A of website Myyachtguardian.com in category: Blog MMO. See more related questions in the comments below.
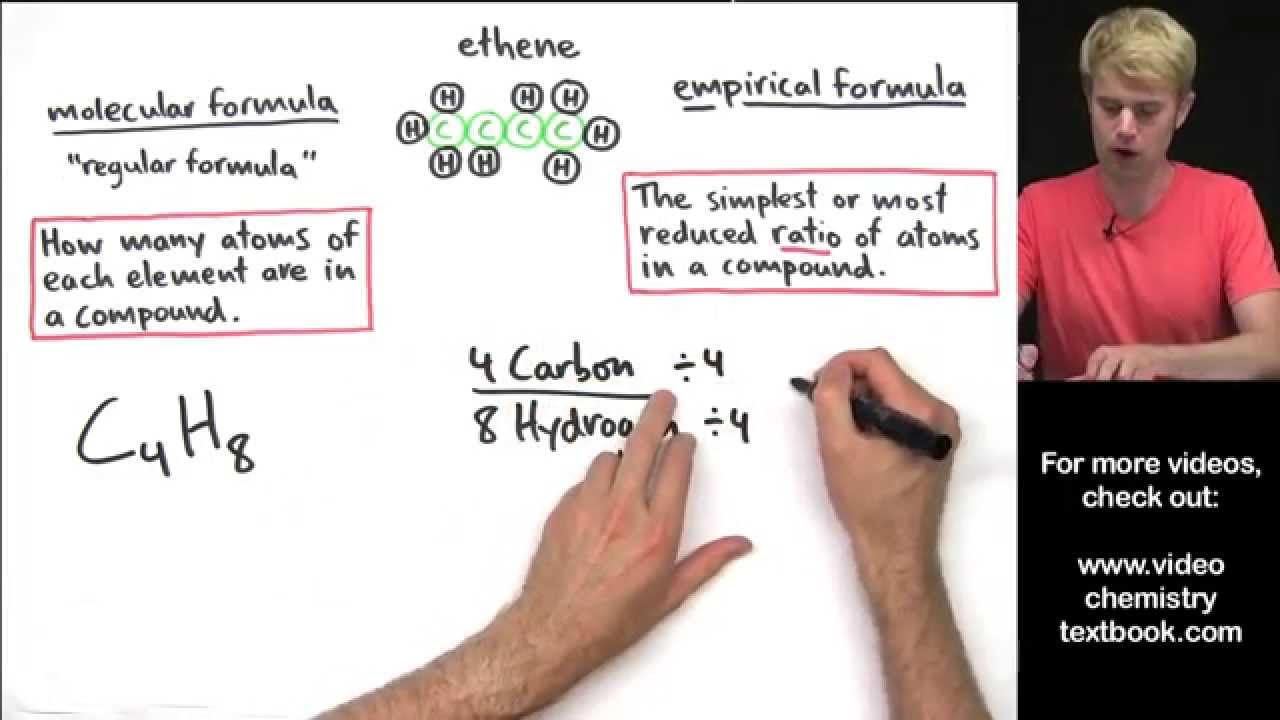
Table of Contents
What is molecular formula and structural formula?
A molecular formula uses chemical symbols and subscripts to indicate the exact numbers of different atoms in a molecule or compound. An empirical formula gives the simplest, whole-number ratio of atoms in a compound. A structural formula indicates the bonding arrangement of the atoms in the molecule.
What information is conveyed when you write the molecular or structural formula for a molecule?
The molecular formula expresses information about the proportions of atoms that constitute a particular chemical compound, using a single line of chemical element symbols and numbers.
Empirical Formula and Molecular Formula Introduction
Images related to the topicEmpirical Formula and Molecular Formula Introduction

Are molecules which have the same empirical formula but have different structures due to arrangement of the atoms in the molecule?
Isomers are compounds with the same molecular formula but different arrangements of atoms.
What do you mean by molecular formula?
Definition of molecular formula
: a chemical formula that gives the total number of atoms of each element in each molecule of a substance — compare structural formula.
What is molecular formula Class 11?
Molecular formula is the actual number of atoms of each element in a compound. It also indicates the kinds of atoms. It is generally derived from the Assays and empirical formula by calculations. Molecular formula can be obtained by multiplying the empirical formula with a whole number coefficient.
What is molecular formula class 10th?
The molecular formula is the expression of the number of atoms of each element in one molecule of a compound. The molecular formula definition is the formula showing the actual number of each atom in a molecule. When the molar mass value is known, the Molecular Formula is calculated by the empirical formula.
Is the molecular formula the same as the structural formula?
What is the difference between molecular formula and structural formula? The molecular formula is just the elements of the molecules with the number of elements. The structural formula is drawing the structure of the molecule.
What information does a molecular formula not reveal?
A molecular formula does not tell you about a molecule’s structure. A variety of diagrams and molecular models can be used to show the arrangement of atoms in a molecule. dioxide shows how the three atoms are arranged in a row. The arrangement of atoms within a molecule is called its molecular structure.
Calculating Molecular Formulas Step by Step | How to Pass Chemistry
Images related to the topicCalculating Molecular Formulas Step by Step | How to Pass Chemistry

What information does a structural formula provide that a molecular formula does not quizlet?
Molecular formulas contain no information about the arrangement of atoms. Because of this, one molecular formula can describe a number of different chemical structures. A structural formula is used to indicate not only the number of atoms, but also their arrangement in space.
What information does a molecular formula provide quizlet?
What information does a molecular formula provide? It provides how many atoms of each type are in a molecule. How is the representative unit of a molecular compound different from the representative unit of an ionic compound? The representative unit of a molecular compound is a molecule.
How do you find empirical and molecular formulas?
STEP 1: Calculate the molar mass of the empirical formula. STEP 2: Divide the given molecular molar mass by the molar mass calculated for the empirical formula. STEP 3: Multiply each subscript by the whole number that resulted from step 2. This is now the molecular formula.
How is molecular formula related to empirical formula?
Molecular formulas tell you how many atoms of each element are in a compound, and empirical formulas tell you the simplest or most reduced ratio of elements in a compound. If a compound’s molecular formula cannot be reduced any more, then the empirical formula is the same as the molecular formula.
How do you find the molecular formula from the empirical formula?
Divide the molar mass of the compound by the empirical formula mass. The result should be a whole number or very close to a whole number. Multiply all the subscripts in the empirical formula by the whole number found in step 2. The result is the molecular formula.
What is molecular formula give one example?
Molecular Formula Definition: An expression which states the number and type of atoms present in a molecule of a substance. Examples: There are 6 C atoms and 14 H atoms in a hexane molecule, which has a molecular formula of C6H14.
How do you find the molecular formula of a Class 9?
- Write the symbol of the positive ion/radical to the left and the negative ion/radical to the right along with their valency number.
- Interchange the valency number of the radicals and shift them to the lower side.
- Example: Ammonium phosphate. NH₄⁺1 PO₄3⁻ (NH₄)₃PO₄
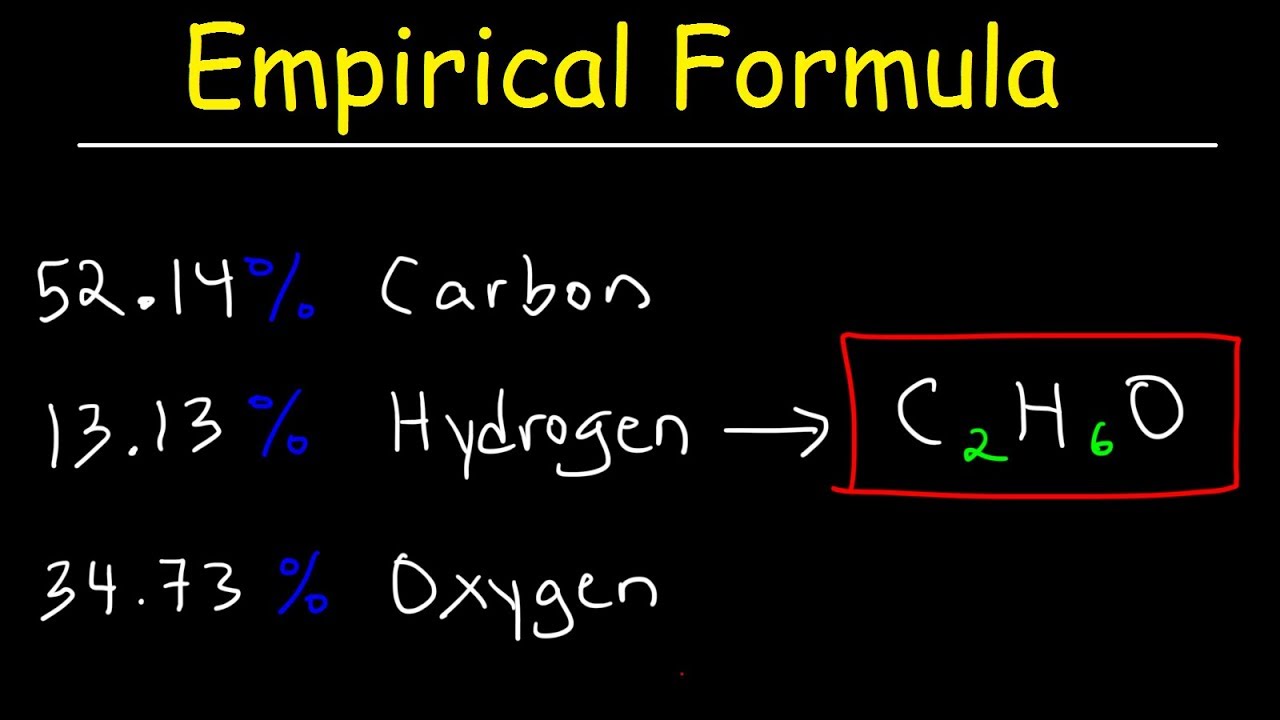
Empirical Formula \u0026 Molecular Formula Determination From Percent Composition
Images related to the topicEmpirical Formula \u0026 Molecular Formula Determination From Percent Composition
What is molecular formula and empirical formula Class 11?
An empirical formula represents the simplest whole number ratio of various atoms present in a compound. Molecular formula shows the exact number of different types of atoms present in a molecule of a compound.
What is a mole Class 11?
A mole is defined as the amount of substance which contains same number of elementary particles (atoms, molecules or ions) as the number of atoms present in 12 g of carbon (C-12).
Related searches
- what is the molecular formula of the molecule
- what is the formula of molecular
- what is the formula of the molecule
Information related to the topic what is the molecular formula for the molecule shown
Here are the search results of the thread what is the molecular formula for the molecule shown from Bing. You can read more if you want.
You have just come across an article on the topic what is the molecular formula for the molecule shown. If you found this article useful, please share it. Thank you very much.

